Lewis Structure Of Krf4
Krf4 lewis structure polar or nonpolar. A step-by-step explanation of how to draw the SeF4 Lewis Dot Structure Selenium TetrafluorideFor the SeF4 structure use the periodic table to find the tot.
What Is The Molecular Geometry Of Sef4 How Is It Determined Quora
How many lone pairs are on the central atom in KrF4.

Lewis structure of krf4. First of all find out the total number of valence. However these structures are helpful in understanding the bonding and valence electron. Krf4 Molecular Geometry KrF4 Lewis.
The electron group geometry of KrF 4 is -A. The sulfur tetrafluoride SF4 is a polar molecule because in SF4 the sulfur atom consists of one lone pair due to which the shape of the molecules becomes asymmetricThe fluorine is more electronegative than sulfur. Determine the total valence count.
Now we start looking at the steps required for drawing the Lewis structure-1. The K r atom is in the center. See Answer Add To cart Related Questions.
CIO2 KRF4 Lewis Structure Total of Valence Electrons electron groups domains on central atom sonance structures Yes or No Molecular geometry Angles around central atom Formal charge on central atom Polar Yes or No 299. The formal charge on an atom in a molecule or molecular ion can be calculated as. Draw the Lewis structure for KrF4 and answer the following question.
What is the hybridization on the central atom. See Answer Add To cart Related Questions. This leads to bending in the structure.
Draw The Lewis Structure Of KrF4 Then Answer The Following Questions. Predict the molecular structure of KrF4 whose Lewis structure is shown below. Also question is what is the Lewis structure for sf4.
The Lewis structure of KrF2 shows that K is surrounded by 3 lone pairs of electrons and forms single bonds with each of the F atoms. Predict The Molecular Structure Of KrF4 Whose Lew. Dsp3 sp sp 3 sp Submit Show Hints.
The Kr-F bond is_____. On the central atom. KrF4 Square planar nonpolar.
How to Draw. This lesson defines Lewis dot structures and explains how to draw them for molecules in step-by-step detail. We count the total number of valence electrons of the whole molecule.
Well also explore polyatomic ions and how to draw Lewis dot structures for them. A lewis dot structure is a drawing of a molecule. The molecular shape of KrF 4 is --A.
Again each molecule has the same number of atoms but a different structure because of differing numbers of lone pairs around the central atom. What is the hybridization on the central atom. A step-by-step explanation of how to draw the KrF2 Lewis Dot Structure Krypton DifluorideFor the KrF2 structure use the periodic table to find the total n.
It is an ionic compound so it would not have a lewis dot structure. It has two lone. Lewis dot structures help predict molecular geometry.
Krf4 Lewis Dot Structure. The F-Kr-F bond angle is approximately_____. A Lewis structure containing one K r atom and four F atoms is shown.
A step-by-step explanation of how to draw the KrF4 Lewis Dot Structure Krypton TetrafluorideFor the KrF4 Lewis structure calculate the total number of val. Draw the Lewis structure for KrF4 and answer the following question. Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule.
Determine the total number of sigma and pi bonds and the hybridization. The valence electrons that participate in forming bonds are called bonding pairs of electrons whereas the electrons that do not participate or form any. KrF4 Square planar nonpolar.
Lewis Dot Structure For Krf4 Lewis Dot Structure of SF4 Sulfur TetraFluoride YouTube How to Draw the Lewis Dot Structure for KrCl4. Dsp3 sp sp2 sp3 d-sp. CIO2 KrF4 Lewis structure Total of valence electrons electron groups Label the functional groups in the molecule.
For each of the following. On each side there is a single bond with an f atom. The bonds formed between two atoms are depicted using lines whereas the valence electrons not forming any bonds are shown by dots.
Step 3 4. How to Draw the Lewis Structure for. KrF4 Square planar nonpolar.
Bf3 can combine with an f. Draw the Lewis structure of KrF 4 then answer the following questions. If you dont remember the valence electrons of a particular atom you can use the periodic table as a reference.
Is KrF4 polar or nonpolar. Dsp3 sp sp 3 sp Submit Show Hints. Draw the Lewis Structure for KrF4 and answer the following question.
To draw the PCL3 lewis structure follow the below instructions. Krf4 Molecular Geometry KrF4 Lewis Structure. September 2010 Chapter 4 Part 2 Lewis Structures Free Photos.
In the Lewis structure of PCL3 there are two chemical. Krf4 lewis structure polar or nonpolar. What is the Hybridization.
Consider The Following Krypton Compounds A 3 Chegg Com

Chapter 8 Bonding General Concepts Chapter 8 Questions

Steps To Draw A Lewis Structure For Exceptions To The Octet Rule Molecular Geometry Octet Rule Chemistry 101

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Teaching Chemistry Chemistry Worksheets Chemistry Education

Pin By Sarah Hawkins On Chemistry Class Molecular Geometry Teaching Chemistry Chemistry Class

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube

Krf4 Lewis Structure How To Draw The Lewis Structure For Krf4 Krypton Tetrafluoride Youtube

Cf4 Lewis Structure Shape Youtube

Draw A Lewis Structure Of Formaldehyde Lewis Chemistry Draw
Lewis Structure Of Cf4 Shefalitayal

Is Krf4 Polar Or Nonpolar Study Com

Ch2cl2 Lewis Structure Molecular Geometry Polarity Dichloromethane Molecular Geometry Molecular Covalent Bonding
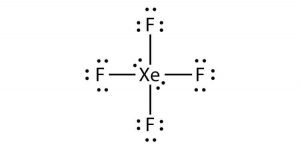
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

Krf4 Lewis Structure How To Draw The Lewis Structure For Krf4 Krypton Tetrafluoride Youtube

Lewis Structure Shows How Valence Electrons Are Arranged Among Atoms In A Molecule Reflects Central Idea That Stability Of A Compound Relates To Noble Ppt Download
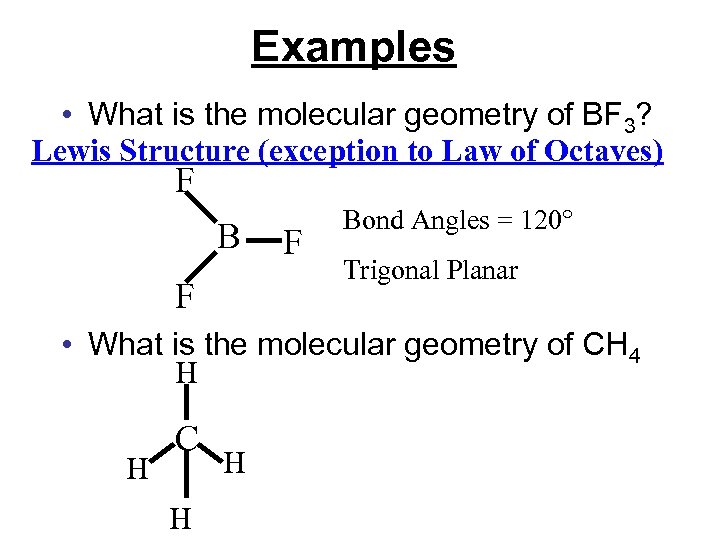
Chapter 9 Molecular Geometry Bonding Theories Molecular


