Pcl3 Lewis Structure Formal Charge
SBr2 Lewis dot structure. The others being POCl3 and PCl3.

Chclo Lewis Structure How To Draw The Lewis Structure For Chclo Youtube
5 73.

Pcl3 lewis structure formal charge. 10 times the heat of evaporation keeps the system at its boiling point and the phosphorus trichloride distills off. What is the hybrid orbital designation for the central atom. How many bonds does N2O.
Valence electrons of Phosphorus Valence electrons of Chlorine. 0 0 0 1 0 1In the left hand2 all structurehe fo mal of charges CO are zero and this structure is favored over the right hand structure. Every atom should be in its least possible formal charge Also every atom in the lewis structure of PCl3 is fulling octet as they are having 8 electrons each after sharing.
Calculate the total number of valence electrons present. Here in this post we described step. To calculate the formal charge in NCl3.
EC LEC 12BE. The Lewis structure of a compound does not deal with the 3-dimensional representation of its elements in space nor its molecular design and geometry. The bromomethane chemical formula is CH3Br.
Please note none of the solutions are using the expanded octet rule or formal charges H 2. Recall that the formula for the formal charge is. In the following computation the formal charge will be calculated on the central carbon atom of the CH3I Lewis dot structure.
Drawing CH3Br Lewis Structure is very easy to by using the following method. The formal charge on the carbon atom of CH3I molecule V. Draw the lewis structure of PCl 3.
How many pi bonds in the formula. Is dative bond present in bcl3. Lastly to ensure the lewis structure is completely correct we need to check the octet of every atom and also their formal charge.
Draw the Lewis structure for the molecule. 7 7 0. EBr LEBr 12BE VE Br Valence electron in a bromine atom of SBr2molecule.
All atoms in BrCl 3 have a formal charge of zero and the sum of the formal charges totals zero as it must in a neutral molecule. Formal charge Valence electrons unbonded electrons 12 bonded electrons We will calculate the formal charge on Nitrogen which is the central atom in the NCl3 lewis dot structure. 7 7 0Cl.
In a Hoechst continuous process molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride. Underneath draw the lewis structure. Total number of valence electrons of PCl3.
A step-by-step explanation of how to draw the POCl3 Lewis Dot Structure Phosphoryl chlorideFor the POCl3 structure use the periodic table to find the tota. This gives the formal chargeBr. The molecule SO3 has two coordinate bonds but that structure is not the most stable form as it carries a formal charge.
To calculate the formal charge on the central carbon atom of the CH3I molecule by using the following formula. Formal charge number of valence electrons - number of lone pair electrons - 05 x number of bonding electrons. How many sigma bonds in the formula.
Lewis Structure of PCl5. Write Lewis structures for the following. H 2 CCH 2.
What is the geometric shape. For the central atom what is the formal charge. Phosphorus Trichloride PCl3 has.
Subtract this number from the number of valence electrons for the neutral atom. The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorusThe heat of reaction ca. What Is The Formal Charge On Phosphorus In The Best Lewis Structure For The PCl3.
After fulfilling the electrons we can see the final lewis structure of PCl3. - Bonds Nonbonding electrons. Calculate the formal charge for the indicated atom.
Chlorine has seven valence electrons but as there are three atoms of Chlorine we will multiply this number by 3. This must be done while considering the relevance of the octet rule and the concepts of formal charges. Note that a Lewis structure for carbon dioxide can be written using a carbon-oxygen single bond on one side and carbon-oxygen triple bond on the other.
Valence electron of nitrogen 5 Non-bonding electrons 2 Bonding electrons 6. Where the lone pair electrons refer to electrons not involved in any form of a bond and bonding electrons refer to electrons involved in a bond. The formal charge on the bromine atom of SBr2 molecule V.
Answer all questions related to the drawing. To calculate the formal charge on the terminal bromine atom of the SBr2 molecule by using the following formula.

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube

Pcl3 Molecular Geometry Shape And Bond Angles Youtube

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
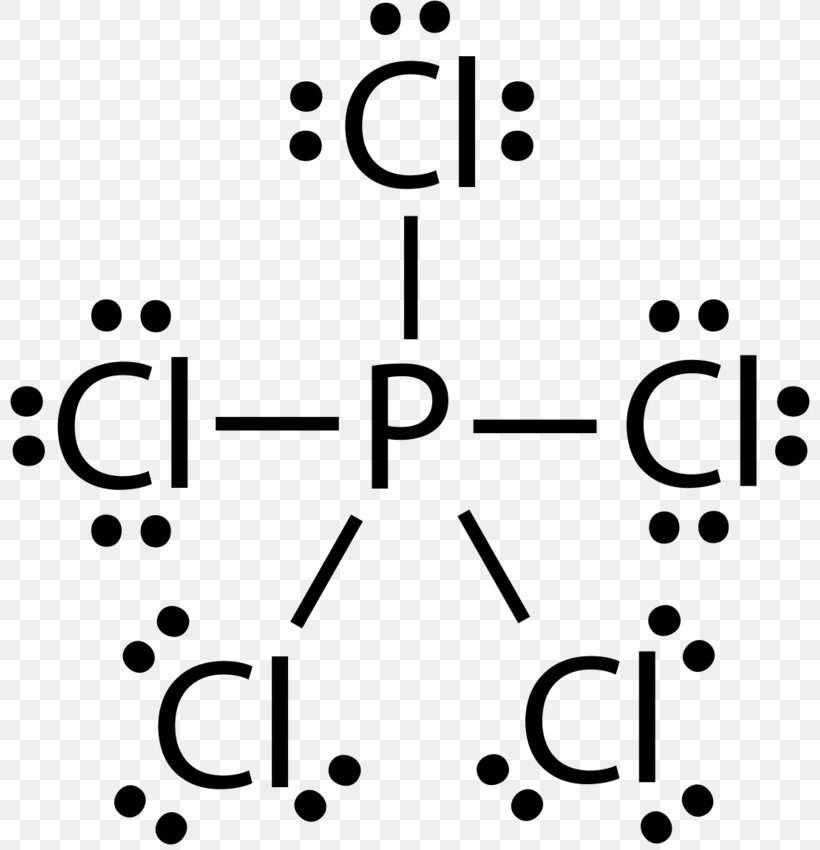
Lewis Structure Phosphorus Trichloride Phosphorus Pentachloride Electron Png 800x850px Lewis Structure Area Atom Black And White

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

How Is The Electron Dot Structure Of Pcl3 Determined Quora

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Ccl2o Lewis Structure How To Draw The Lewis Structure For Ccl2o Youtube
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Resonance Structures For No3 Nitrate Ion Youtube
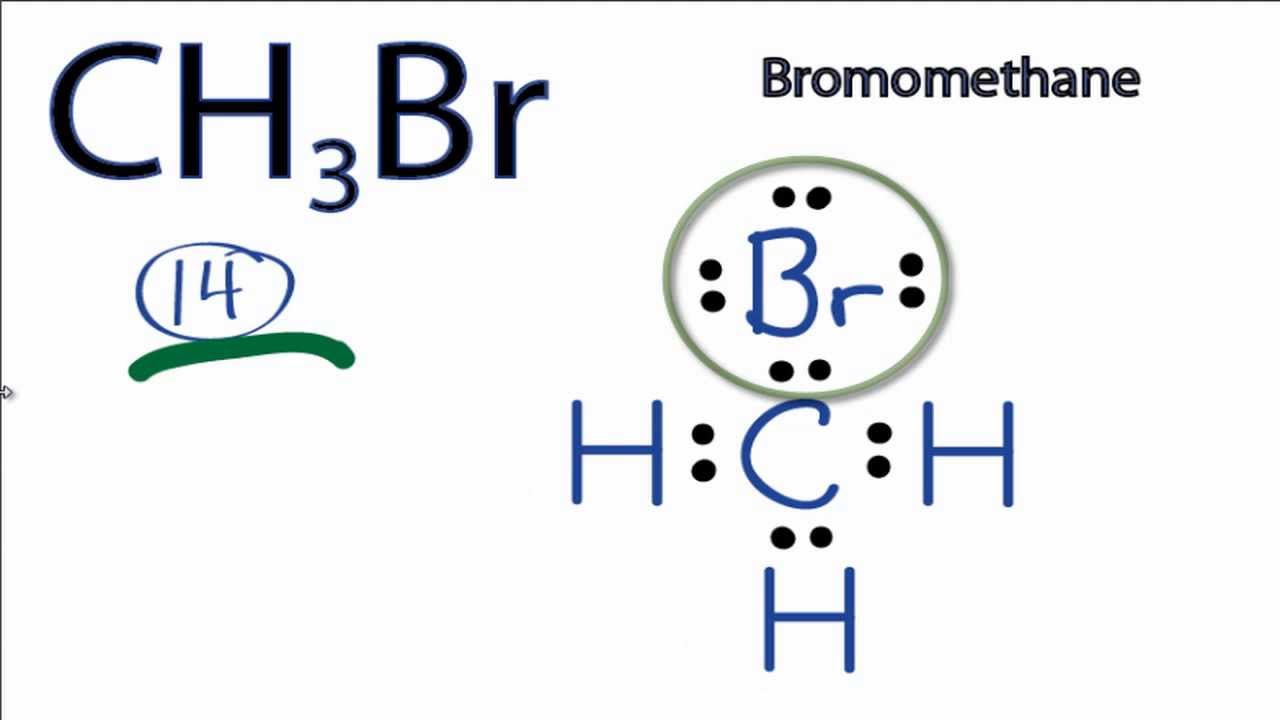
Ch3br Lewis Structure How To Draw The Lewis Structure For Ch3br Bromomethane Youtube
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Bcl3 Lewis Structure How To Draw The Lewis Structure For Bcl3 Youtube

Bcl3 Lewis Structure 3d What Is The Molecular Geometry Of Bcl3 Draw Its Vsepr And Lewis Structure Socratic Note That As Several Viewers Have Pointed Out At 2 53 In The
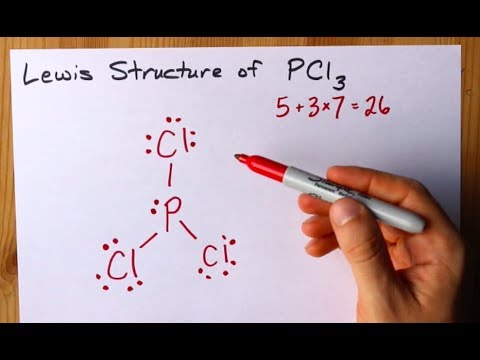
How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube