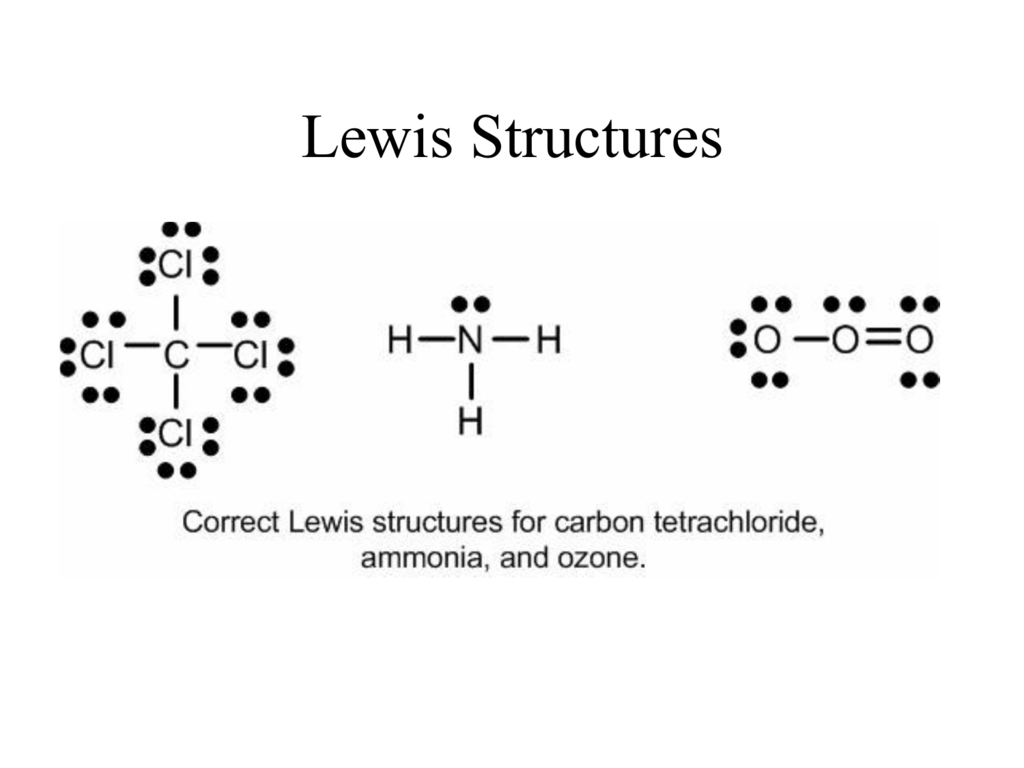
Pcl3 Lewis Structure Valence Electrons
To draw the lewis structure first of all we need to sum up the valence electrons of all the atoms. Total electron pairs are determined by dividing the number total valence electrons by two.

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
5 73.

Pcl3 lewis structure valence electrons. The electron geometry is the arrangement of electron groups As I have discussed in the PCL3 lewis structure that there is a total of 26 valence electrons in the PCL3. Chlorine has seven valence electrons but as there are three atoms of Chlorine we will multiply this number by 3. Cl-P-a Each Cl atom has 6 valence electrons as does the P atom so the Lewis structure for PClz will include 24 electrons.
Valence electrons of Phosphorus Valence electrons of Chlorine. The geometry of the CH3I molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory VSEPR Theory which states that molecules will choose the CH3I geometrical shape in which the. Calculation of total valence electron of HBr molecule Choose the atom with the least electronegative value atom and insert it in the center of the molecular geometry of HBr.
Total valance electrons pairs σ bonds π bonds lone pairs at valence shells. Selection of center atom. A total of 28 valence electrons.
Lewis Structure of PCl3 and Hybridization of PCl3. 8cdot4 32 Total sharedbonding electrons. The sulfur atom in the molecule gets only 8 electrons around its molecular structure.
Now lets move on to the lewis structure of PCl3. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs. -Ncl3 will have Sp3 hybridization as there is one lone pair and 3 bonds of N and cl so total steric no.
Which statement correctly describes the number of valence electrons to include. Total valence electrons pairs. C Number of electron domains.
Steps of drawing PCl 3 lewis structure Total number of electrons of the valance shells of PCl 3. For PCl 3 Total pairs of electrons are twenty 402 in their valence shells. Here Phosphorous 5 valence electrons Chlorine 7 valence electrons 3 Cl 73 21 So total valence electrons 26.
PCl3 is a covalently-bonded molecule which is officially named phosphorus trichloride. SBr2 Lewis structure diagram we always begin by introducing valence electrons from the central sulfur atomin step1. 1 x P 1 x 5 5.
7 for Chlorine but we have three Chlorines. 32-266 In â In the Lewis structure for PCl 3 there are a total of 26 valence electrons. 7cdot2 6cdot2 26 Total electrons needed for octetsdoublets.
Total electron pairs are determined by dividing the number total valence electrons by two. I is the least electronegative put that in the center and then Chlorines will go around it. The bonding domains for PCl3 are shown in the Lewis structure below.
The properly way to determine the Lewis structure based on this example is. What is the Lewis structure for ICl3. It has three chlorine atoms around a single phosphorus atom and that phosphorus also has a lone pair of electron which complete its octet.
5f romP37f romCl 26 5 f r o m P 3 7 f r o m C l 26. This is the ICl3 Lewis structure. For ICl3 we have 7 valence electrons for Iodine.
If you mean how to work out the Lewis structure for PCl3 first count all available valence electrons as follows. PCl3 lewis structure In this lewis structure of PCl3 center phosphorus atom has made three single bonds with three chlorine atoms. Total outermost valence shell electrons available for HBr Lewis structure dot structure 711 8 valence electrons in HBr.
Total number of valence electrons of PCl3. K is normally drawn with zero dots because K just lost its only valence electron although K of course has 8 valence electrons. As a result wrap around the central sulfur atoms bond pair valence electrons first see figure for step1.
The lewis structure of PCl3 can be explained as follows. Here are the details how it becomes 26. KClO 4 is ionic.
PCl 3 phosphorus is in the middle with one lone pair and is surrounded by single bonds to the three chlorines each with 3 lone pairs. There are only two elements in phosphorus trichloride. Phosphorus Trichloride PCl3 has.
Each Cl atom has 7 valence electrons and the P atom has 3 valence electrons so the Lewis structure for PClz will include 24 electrons. Its lewis structure is shown below. The CH3I Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the CH3I molecule.
Phosphorus 5 valence electrons Chlorine 7 valence electrons. A Lewis structure of PCl3 P C l 3.

Ch3cl Lewis Structure Chloromethane In 2021 Molecules Lewis Methylation

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Cf2cl2 Lewis Structure How To Draw The Dot Structure For Cf2cl2 Dichl Drawings Dots Lewis

Lewis Dot Structure Easy Hard Science
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Hybridization Of Ch3cl Chloromethane In 2021 Molecules Lewis Chemical Formula

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium

Chclo Lewis Structure How To Draw The Lewis Structure For Chclo Youtube

Ch2cl2 Lewis Structure Dichloromethane In 2021 Molecules Math Equations Hydrogen Atom
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Http Www Youtube Com Watch V Qojoaosk5ui Lewis Chemistry Math Equations

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
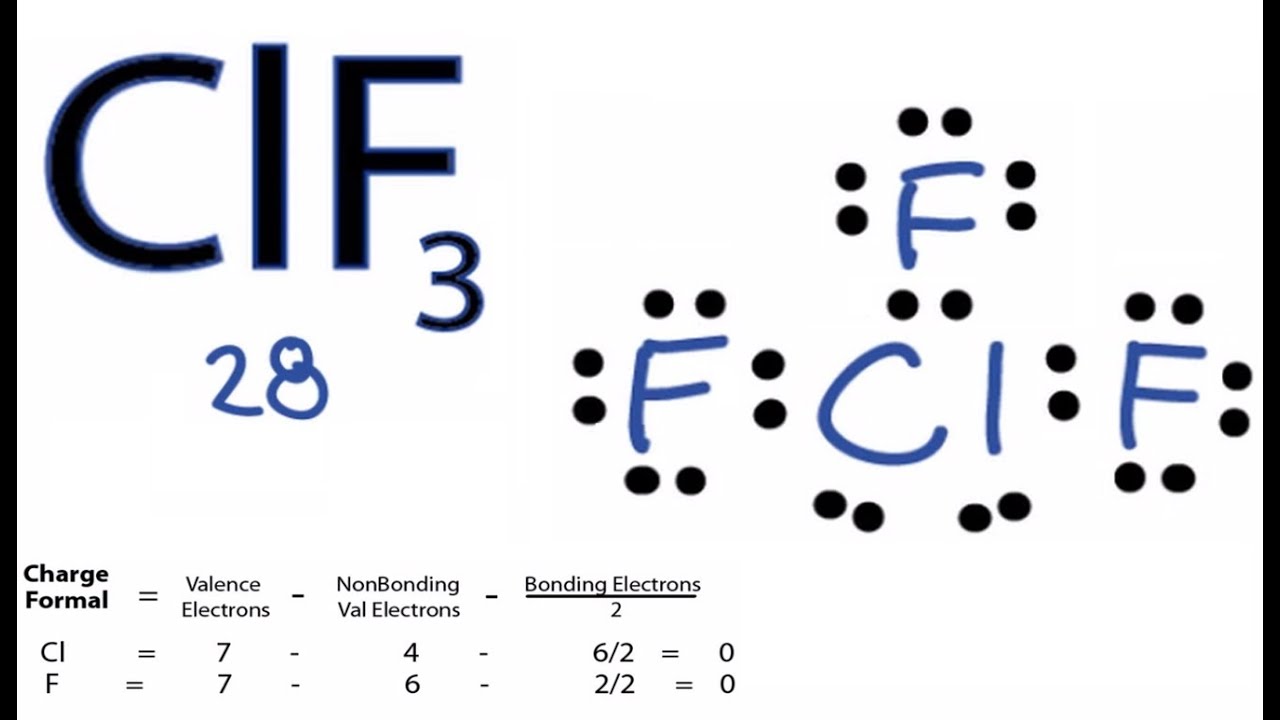
Clf3 Lewis Structure How To Draw The Lewis Structure For Clf3 Youtube

Pcl3 Lewis Structure How To Draw The Dot Structure For Pcl3

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules