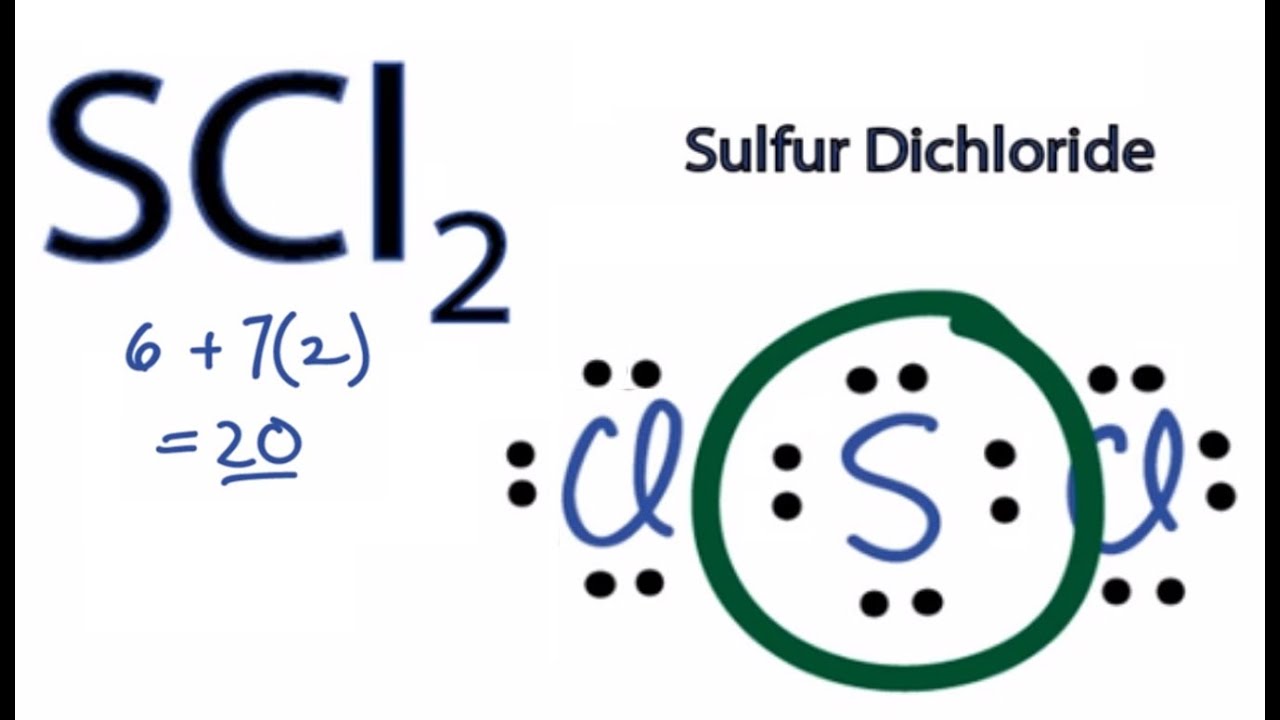
Sci2 Lewis Structure
The molecular geometry of SCl2 simply determined by the coordination number which is equal to 2. So total valence electrons 18.
It determines the number of valence electrons and the electrons taking participation in forming the bonds of the molecule.

Sci2 lewis structure. So we have eight valence electrons. The Sulphur atom has sp3 Hybridization and the bond angle of F-S-F is 98 degrees. None of these require pi-bonding which is the method of formation for double and triple bonds.
None of these require pi-bonding which is the method of formation for double and triple bonds. There are 2 oxygen atoms in the compound thus 62 12. For the last one we have ch four.
According to Lewis structure SOCl 2 has three atoms that are attached to central Sulfur that include two Chloride and Oxygen. The Lewis Structure of SCl2 sulfur dichloride has one sulfur single-bonded two each of two chlorine atoms. The simple Lewis structure that is carbon in the center with eight valence electrons all used as bonding.
Draw Lewis structures for each of the following molecules. The Lewis Structure of SCl2 sulfur dichloride has one sulfur single-bonded two each of two chlorine atoms. Thus the hybridization of SCl2 is sp3.
Carbon has four each. The formal charge on the sulfur atom of SBr2 molecule V. SBr2 Lewis dot structure.
And the valence electrons of oxygen 6. There are two lone pairs of electrons on the Sulphur atom which makes the geometry of the molecule bent. Hydrogen really needs to.
To calculate the formal charge on the central sulfur atom of the SBr2 molecule by using the following formula. Jan 16 2015 SCl2 has a bent molecular geometry with bond angles of approximately 103 and a bond lenght of 201 pm. First draw the Lewis dot structure.
Second place the valence electron on the iodine and hydrogen atoms. Hydrogen has one valence electron but there are four of them. List of lewis structures.
First the valence electrons are placed around the carbon atom. Paris bonding one hydrogen to carbon. SCl2 Lewis and Geometrical Structure The lewis structure of a molecule is also known as its electron dot structure.
Note that Sulfur is the least electronegative atom in the SCl2 Lewis structure and is therefore placed in the center. Indicate the total number of valence electrons in the following ions. First of all find out the total number of valence electrons in the C2H4 using the periodic table.
SF 2 has a simple Lewis structure in which the Sulphur atom is in the centre forming single bonds with both the Fluorine atoms. SiCl4 NF3 CO2 HNO SCI2 H2O 92 Molecules and Charge 2. The structure of thionyl chloride showing the trigonal pyramidal geometry is shown below.
NHA PO3- NO2 H3O here to search 13 WRITTEN ASSIGNMENT 3. Hence the molecular geometry of SCl2 is bent. Sulfur is the central atom in the Lewis structure of SCl2 has a steric number equal to 4 hence electron geometry of SCl2 is tetrahedral.
The sulfur atom here has two bonding pairs shown as horizontal lines and two lone pairs shown as two dots for each pair. The sulfur atom here has two bonding pairs shown as horizontal lines and two lone pairs shown as two dots for each pair. SCl2 consists of a single Sulfur atom surrounded by two Chlorine atoms.
To draw the SCl2 Lewis structure follow the below instructions. In SCl2 the sulfur is in group 6 or 16 in the periodic table and has six valence electrons. In its most stable state Sulfur acts as the central atom and forms two covalent bonds with the Chlorine atoms.
In the following structures one atom has a nonzero formal charge. It also possesses two lone pairs. Science Chemistry Lewis structure Draw the molecule SCl2 by placing atoms on the grid and connecting them with bonds.
For the SCl2 Lewis structure use the periodic table to find the total number of valence electrons for the SCl2 molecule. After drawing the skeletal structure we can see that none of the atoms can fulfill their octet with single bonds. In SO2 the sulfurs valence electron 6.
In the Lewis structure of SCl2 the use of a double bond is necessary. In this post we discussed the method to construct the CH3I Lewis structure. If we talk about the lewis structure of SCl2 we need to know about the valence electrons of SCl2.
ES LES 12BE. Now lets see the lewis structure of SO2. Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero.

The Lewis Diagram For Scl2 The Electron P Clutch Prep

Ap Chemistry Covalent Bonding Lewis Structures And The Octet Rule Youtube
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

How To Draw Lewis Structure For Scl2 Drawing Easy
Bond Angle Of Scl2 Lewis Structures
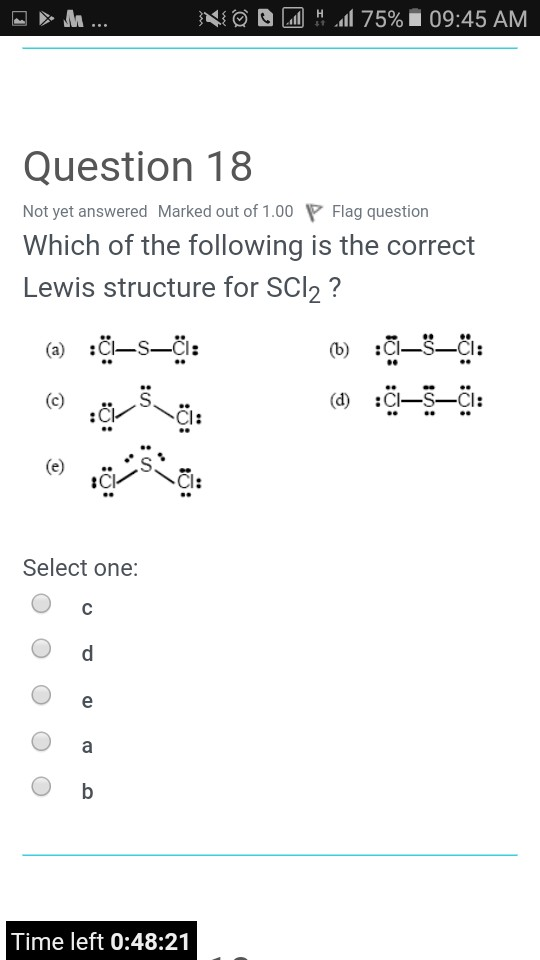
Noc2075 1 09 45 Am Question 18 Not Yet Answered Chegg Com

Does Scl2 Have A Dipole Moment Clutch Prep

Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

Bonding Molecular Shape Structure By Dr Fawaz Aldabbagh
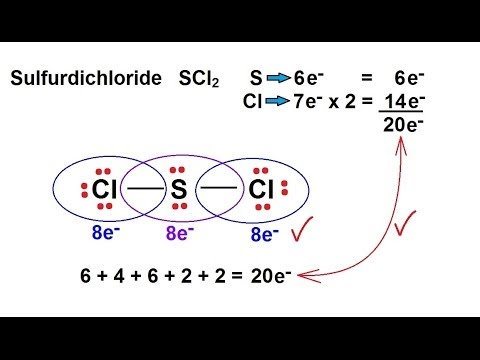
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube

Using The Octet Rule Write The Lewis Formulas For A Scl2 B Ccl4 C Nf3 And D Ch2c Ch3 2 Brainly Com

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep
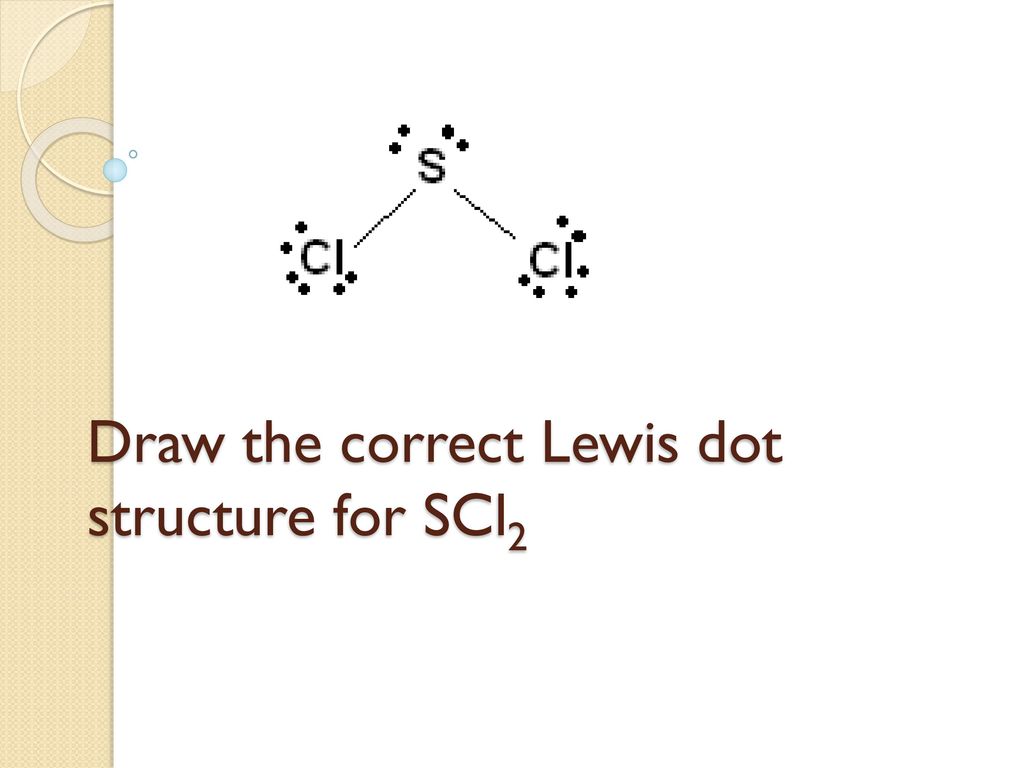
Types Of Bonding And Lewis Structures Ppt Download

Does Scl2 Have A Dipole Moment Clutch Prep
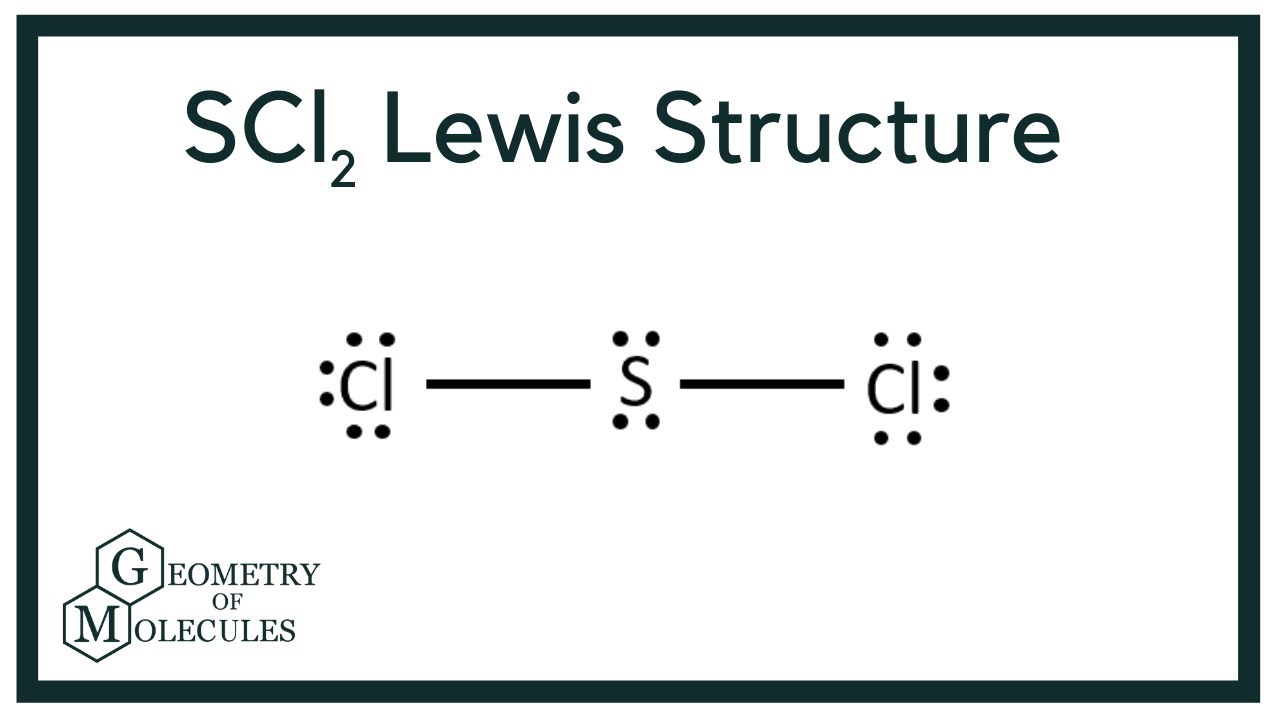
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
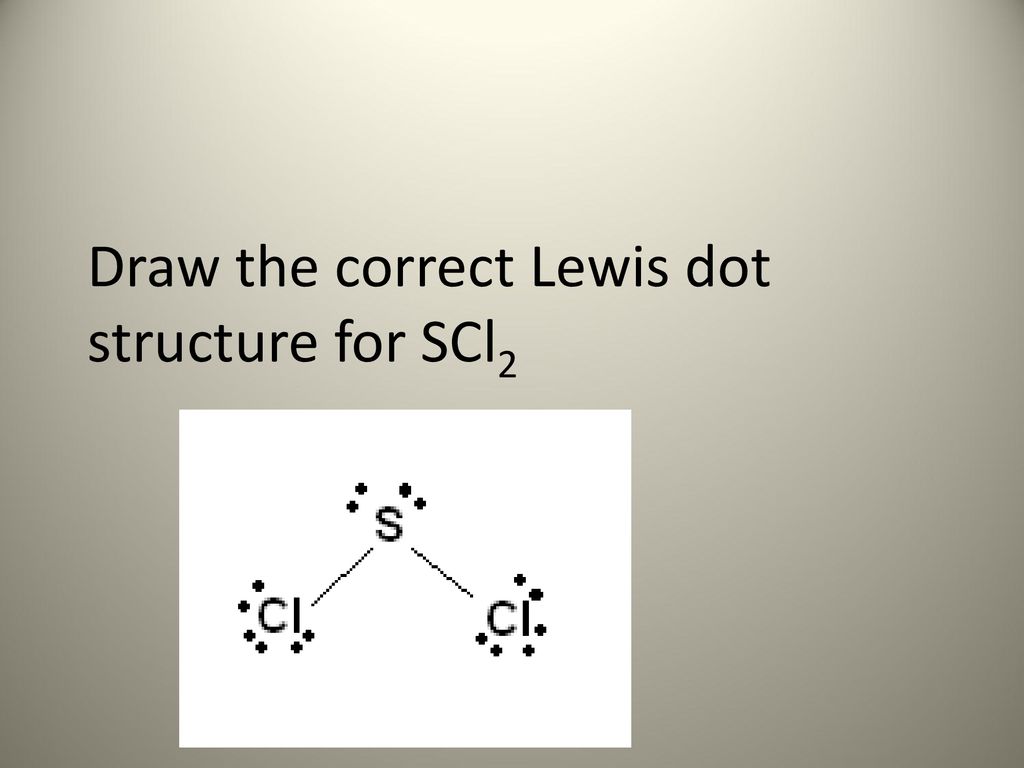
These Are All The Review Questions From Our 5 Exams Ppt Download