Scl2 Lewis Structure Name
As Cl2 lewiss structure only contain two atoms that are similar so you can assume any of one is central and the other one is the outer atom. Therefore SCL2 is a polar molecule.
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube
The structure of Lewis HSO there are three pairs of communication and one lone pair on the S atom so SN No 4.
Scl2 lewis structure name. The second step is to add valence electrons to the one hydrogen atom and the final step is to combine the step1 and step2 to get the HBr. Start with the molecules Lewis structure which is drawn like this. It might appear from the two dimensional drawing that the dipoles of the two S-Cl bonds should cancel one another to yield a nonpolar molecule.
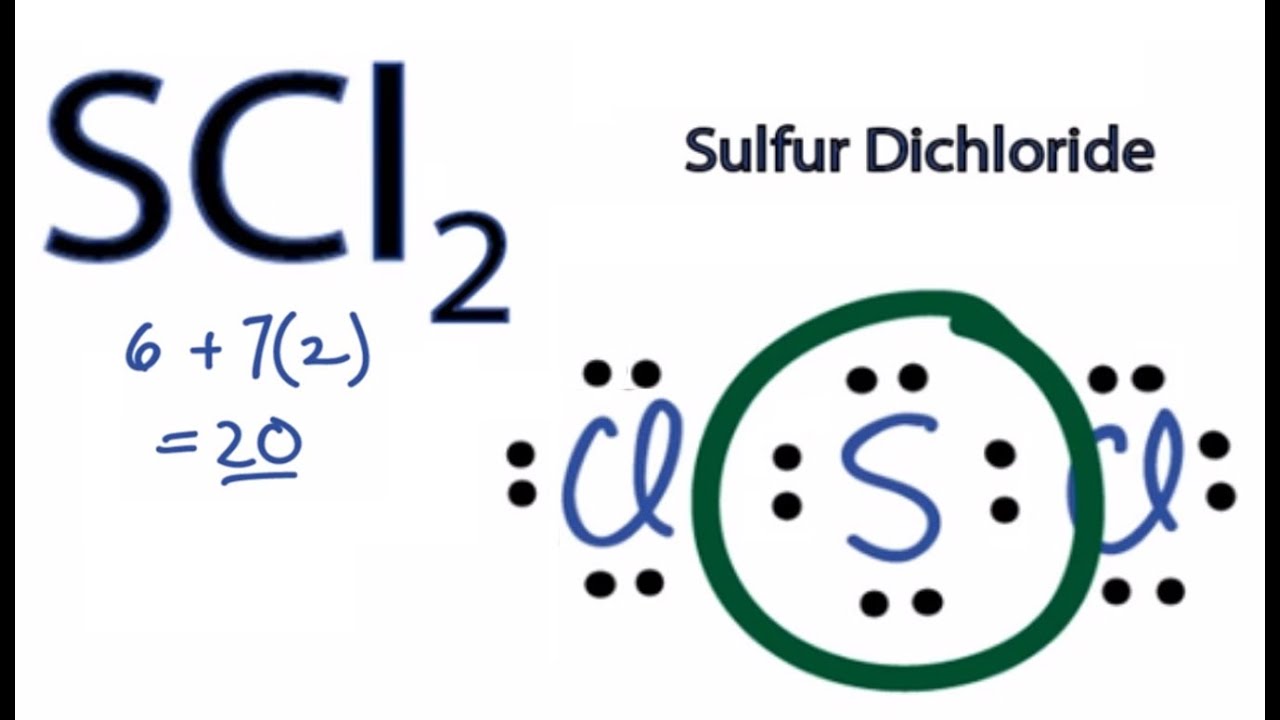
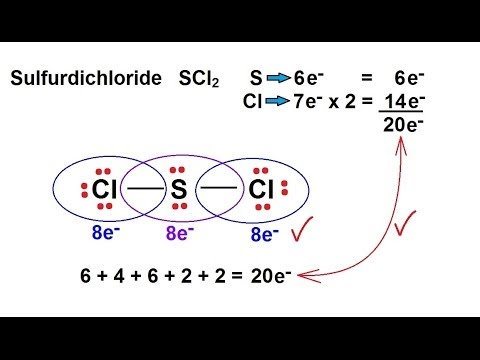
Expert Answer 100 3 ratings. Sulfur chloride SCl2 Chlorine sulfide. For the SCl2 Lewis structure use the periodic table to find the total number of valence electrons for the SCl2 molecule.
Lorem ipsum dolor sit amet consectetur adipisicing elit. Note that Sulfur is the least electronegative atom in the SCl2. Determine the Lewis structure VSEPR and the name of the shape for the following.
Second place the valence electron on the iodine and hydrogen atoms. Now we are left with 12 valence electrons more. I cant draw it but each atom has 6 electrons around it.
The sulfur atom here has two bonding pairs shown as horizontal lines and two lone pairs shown as two dots for each pair. Sulfur being the less electronegative atom than chlorine atom is placed at the center in lewiss diagram and chlorine is spaced evenly around it. In this post we discussed the method to construct the CH3I Lewis structure.
The chemical formula SCl2 represents Sulfur Dichloride. WHAT IS MOLECULAR SHAPE OF OF2. First the valence electrons are placed around the carbon atom.
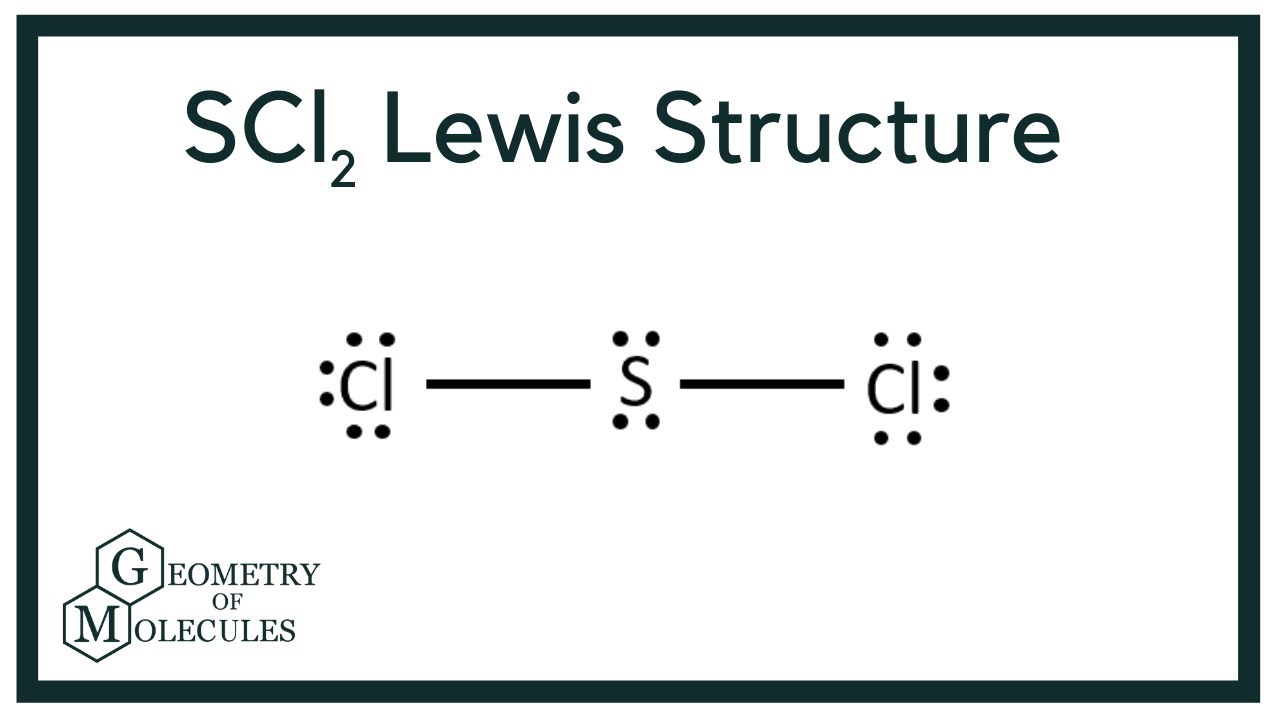
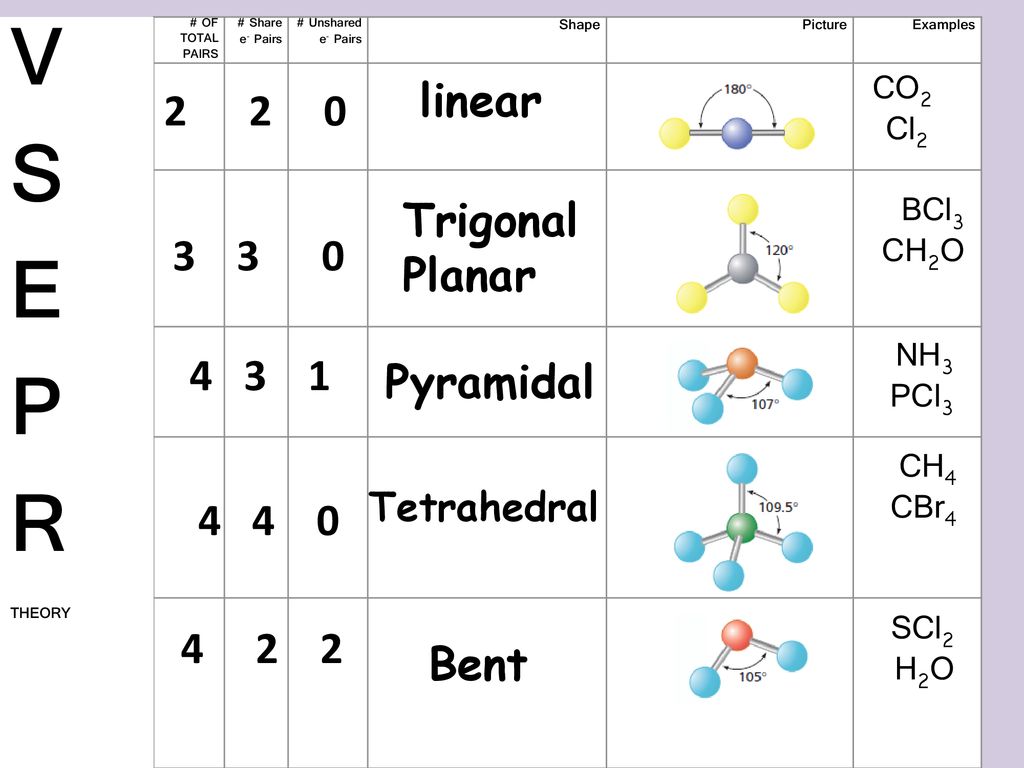
It is the simplest form of Sulfur Chloride and exists as a cherry-red liquid at room temperature. The Lewis Structure of SCl2 sulfur dichloride has one sulfur single-bonded two each of two chlorine atoms. SCl2 has a bent molecular geometry with bond angles of approximately 103 and a bond lenght of 201 pm.
In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. WHAT IS THE LEWIS STRUCTURE OF OF2. WHAT IS THE LEWIS STRUCTURE OF OF2 2.
A step-by-step explanation of how to draw the SCl2 Lewis Structure Sulfur Dichloride. A XeF4 b SCl2 By signing up youll get thousands. WHAT IS MOLECULAR SHAPE OF OF2.
There is two lone pair present on the central atom and this central atom attached to two bonded pair in SCl2 lewis structure. A brief explanation of SOCl2 molecular geometry including a description of SOCl2 communication angles. The sulfur dichloride SCl2 is a polar molecule because the molecule is bent.
The sulfur dichloride SCl2 is a polar molecule because SCl2 has lone pairs of electrons in the central atom of sulfur which results in a bent structure and the molecules include a permanent dipole. The first step is to sketch the Lewis structure of the HBr molecule to add valence electrons around the bromine atom. Key Points To Consider When Drawing The HBr Electron Dot Structure.
It is important to remember that Lewis structures are not meant to convey geometry so it would be wrong to assume that the molecule is linear just by looking. 14 2 12 valence electron. This problem has been solved.
It is obtained via chlorination of S2Cl2 whose impure presence is then distilled using PCl3 to give pure Sulfur Dichloride. Jan 16 2015. This cherry-red liquid is the simplest sulfur chloride and one of the most common.
A three-step approach for drawing the HBr Lewis structure can be used. Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom. For the SCl2 Lewis structure use the periodic table to find the t.
Sulfur dichloride is the chemical compound with the formula SCl 2. It is used as a precursor to organosulfur compounds. WHAT IS 3-D MODEL OF OF2 AND SCl2.
Use your molecular models to explain why this molecule is polar. The Lewis Structure for SCl2 is given. It wont allow me to upload images.
None of these require pi-bonding which is the method of formation for double and triple bonds. Place remaining valence electrons starting from outer atom first. SCl2 lewis structure contains one sulfur and two chlorine atom.
WHAT IS 3-D MODEL OF OF2 AND SCl2 3.
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Khurak
Scl2 Lewis Structure Sulfur Dichloride Youtube

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization

Ch2cl2 Lewis Structure Dichloromethane In 2021 Molecules Math Equations Hydrogen Atom
Lewis Structure Of Scl2 And Hybridization Of Scl2 Lewis Structures

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity

Xef4 Molecular Geometry Bond Angles Electron Geometry Xenon Tetrafluoride In 2021 Molecular Geometry Molecular Geometry

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Is Pf5 Polar Or Non Polar Phosphorus Pentafluoride In 2021 Chemical Formula Molecules Phosphorus

Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules
7th Inning Stretch Pcl3 2 Scl2 3 Cs2 4 Ch4 Ppt Download

Is Scl2 Polar Or Nonpolar Techiescientist

What Is The Name Of The Hybrid Orbitals Us Clutch Prep
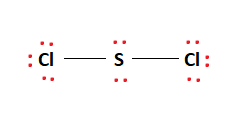
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Is Nh3 Polar Or Nonpolar Vsepr Theory Molecules Polar