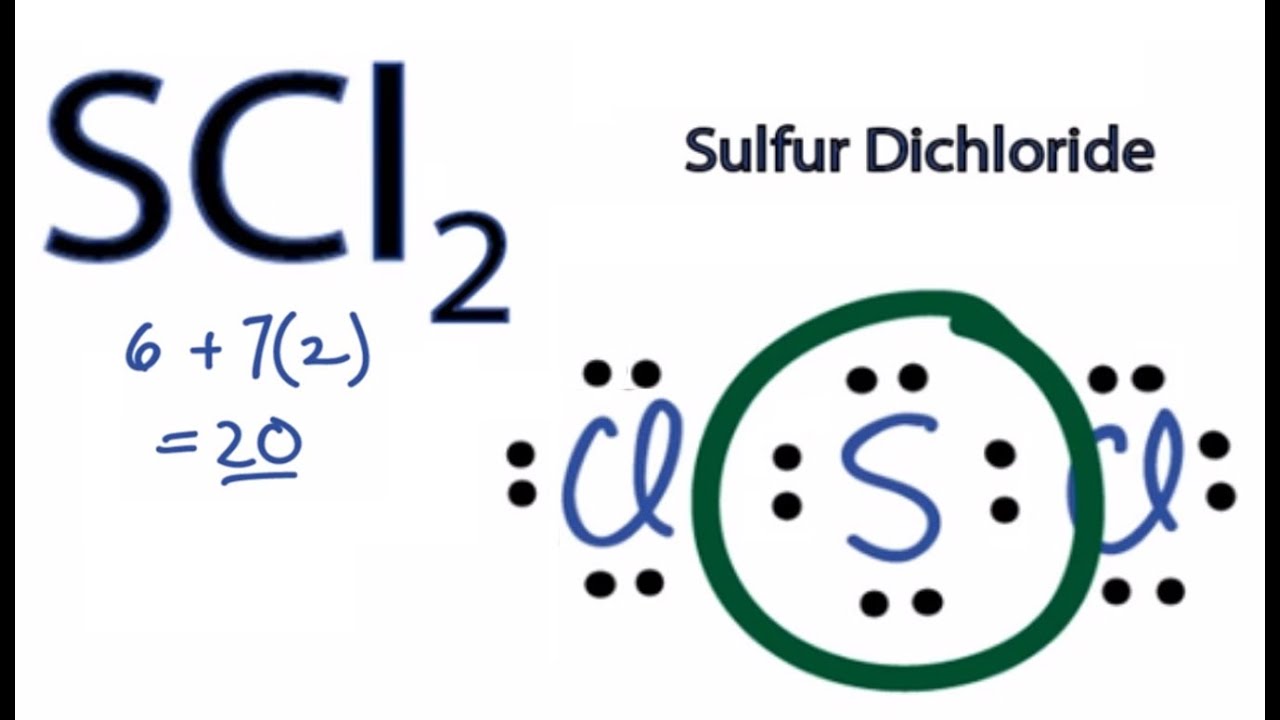
Scl2 Valence Electrons
It consists of K and ClO2- There are 20 valence electrons on the chlorite. Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
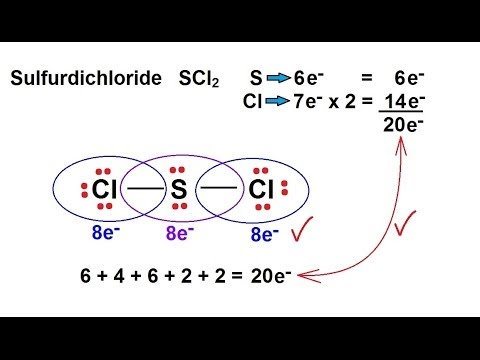
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube
Total valence electrons 14 6 20 S is the central atom and has two sets of bonding electrons - one set for each bond to Cl.

Scl2 valence electrons. Lets do the SCl2 Lewis structure. There are 8 electrons around each atom with two nonbonded electron pairs on the Cl and three nonbonded electron pairs on each Oxygen. Our goal however isnt predicting the distribution of valence electrons.
Sulfur is the least electronegative and therefore is placed in the center of the skeletal structure. By drawing the Lewis Structure accurately we can determine the shape and hybridization of SCl2 as well. Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
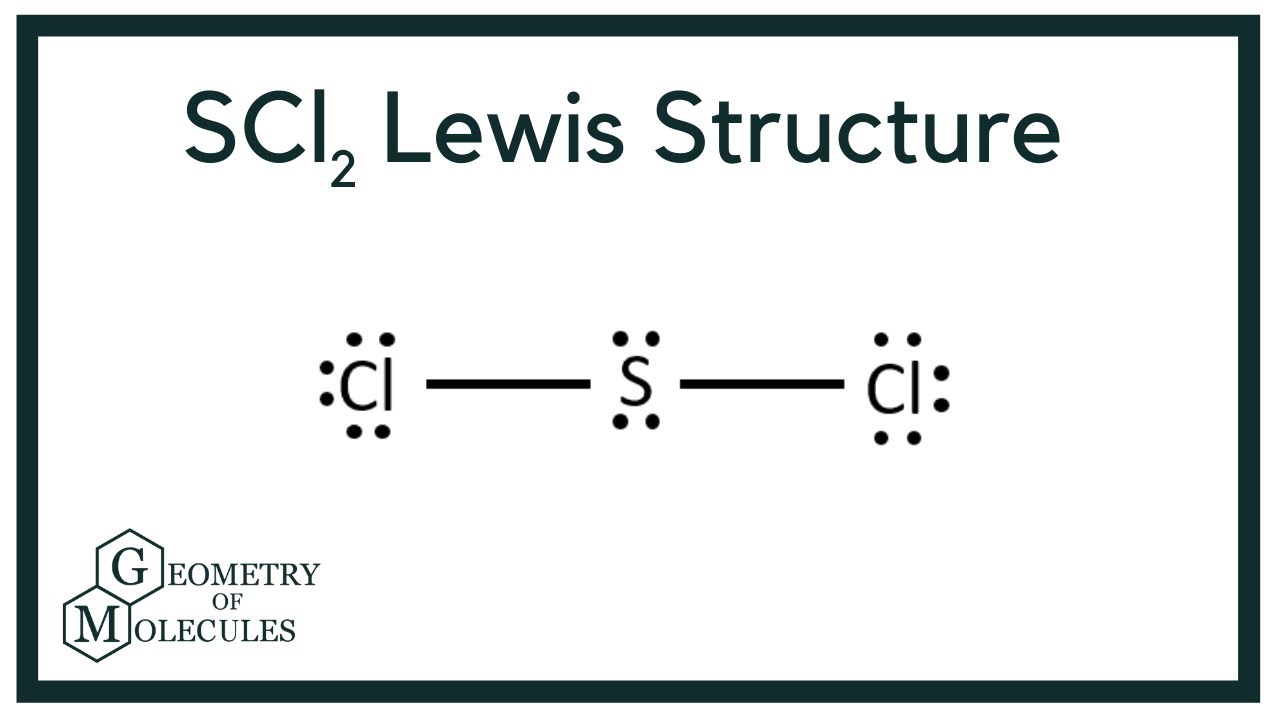
Each Cl has 6 electrons. Now the total number of valence electrons available in SCl2 is given by. Note that Sulfur is the least electronegative atom in the SCl2 Lewis structure and is therefore placed in the center.
Both chlorine atoms form covalent bonds with the sulfur atom leaving behind the two lone pairs on sulfur atom. Chlorine group 7 or 17 has 7. We subtract 10 electrons to account for the five bonds in the skeleton leaving 30 electrons to distribute.
The electrons that occupy the outer most shell of an atom are called valence electrons. We will now begin placing the 20 valence electrons available to us in accordance with the. In SCl2 the sulfur is in group 6 or 16 in the periodic table and has six valence electrons.
Sulfur is the middle element of the molecule with 6 electrons in its outermost valence electron shell while chlorine atom is the outermost valence electron shell with 7 electrons and one electron missing in the shell to complete its octet. So 4 valence electrons. In this video were going to apply VSEPR theory to six electron clouds so if our goal is to find the shape of the sulfur hexafluoride molecule once again we start with our dot structure so sulfur is in group 6 on the periodic table so six valence electrons fluorine is in group seven so seven valence electrons but I have six of them so seven times six gives me 42 and 42 plus six gives me 48.
On the periodic table Sulfurgroup 6 or 16has 6 valence electrons. For the SCl2 Lewis structure we have a total of 20 valence electrons. The lone pairs cause repulsion with bond pairs due to which the S-Cl bonds face force in the downward direction and the shape of molecules becomes bent like that H2O water molecule.
The chlorine is in group 7 or 17 and has seven valence electrons but we have two chlorine so. The valence electrons on the central atom in both NH 3 and H 2 O should be distributed toward the corners of a tetrahedron as shown in the figure below. By writing an electron configuration Youll be able to see how many electrons occupy the highest energy level.
We then line up the two Chlorine atoms on either side of the Sulfur atom. As a result of this above explanation the SCl2 molecule contains a total of 20 valence electrons. For the SCl2 Lewis structure use the periodic table to find the total number of valence electrons for the SCl2 molecule.
It is to use this distribution of electrons to predict the shape of the molecule. They are important because they determine how an atom will react. First of all find out the total number of valence electrons in the C2H4 using the periodic table.
Therefore we have a total of 20 valence electrons present in the SCl2 molecule. A step-by-step explanation of how to draw the XeF5 Lewis Dot StructureFor the XeF5 structure use the periodic table to find the total number of valence. 6S 14Cl 20 valence electrons.
Cl is in the center bonded to the 2 Oxygen atoms. But we have two Chlorines so lets multiply that by 2. This results in 4 electrons used.
The Lewis Structure of SCl2 sulfur dichloride has a sulfur atom which brings six valance electrons bonded two chlorine atoms which each bring seven valence electrons.
Valence Shell Electron Pair Repulsion

What Is The Lewis Structure For Scl2 Study Com

Is Scl2 Polar Or Nonpolar Techiescientist

Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

What Is The Electron Pair Geometry And Mol Clutch Prep
Bond Angle Of Scl2 Lewis Structures
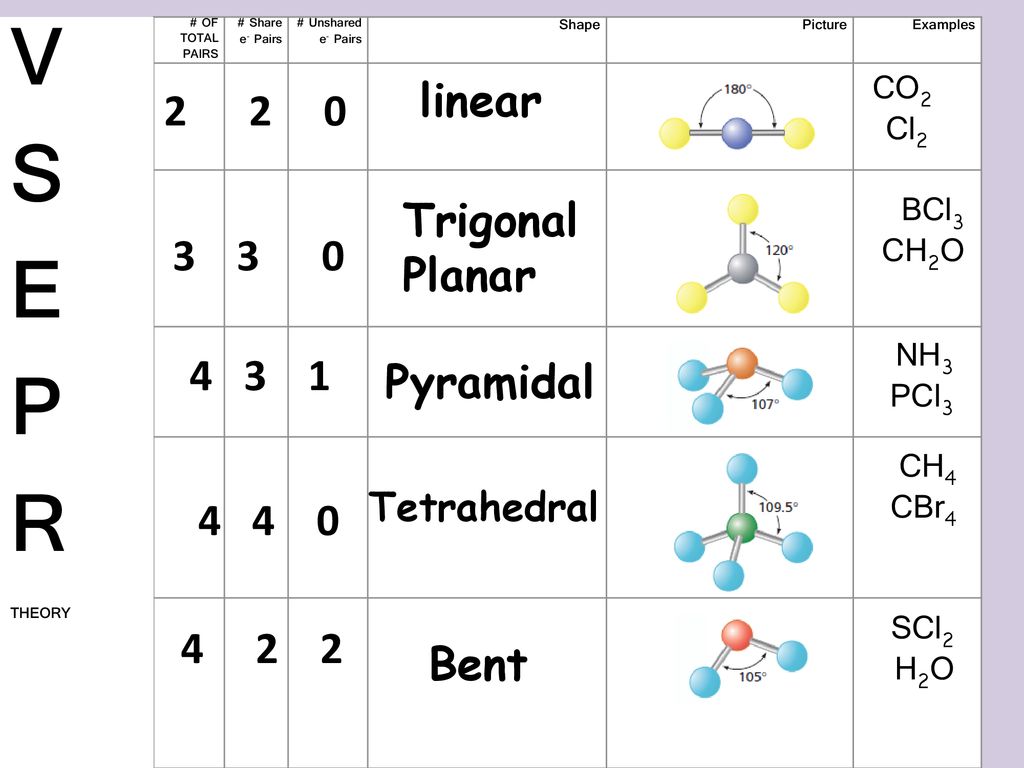
7th Inning Stretch Pcl3 2 Scl2 3 Cs2 4 Ch4 Ppt Download

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity

Does Scl2 Have A Dipole Moment Clutch Prep

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity

Does Scl2 Have A Dipole Moment Clutch Prep

What Is The Name Of The Hybrid Orbitals Us Clutch Prep
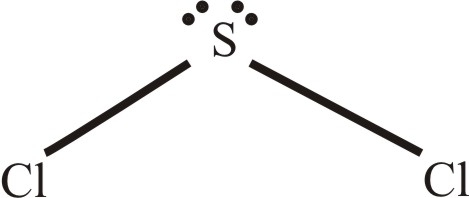
Solved Predict The Geometry Of Sulfur Dichloride Scl2 And The H Chegg Com

The Lewis Diagram For Scl2 The Electron P Clutch Prep
Scl2 Lewis Structure Sulfur Dichloride Youtube
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Using The Octet Rule Write The Lewis Formulas For A Scl2 B Ccl4 C Nf3 And D Ch2c Ch3 2 Brainly Com