Structure De Lewis Pcl3
Therefore P 6n 2 V 6 5 2 32 0 So there is no double bond. Draw the lewis structure of PCl 3.
Exercise I On Drawing Lewis Structures And Adding The Valence Electrons Solutions
Now lets move on to the lewis structure of PCl3.
Structure de lewis pcl3. Añade tu respuesta y gana puntos. Electron dot structures of POCl3. Now the central atom is generally the least electronegative atom or atom with the.
How many pi bonds in the formula. The lewis structure of PCl3 can be explained as follows. Cl tiene 7 electrones.
Percy we have PCL three. So we have eight valence electrons. The straw Lewis structures for each of the following molecules for a H two is a little structure h each for be hbr.
It is a toxic compound but is used in several industries. The resonance Lewis electron dot structures of POCl3 are as follows. Once we know how many valence electrons there are in PCl5 we can distribute them around the central atom and attempt to.
How to Draw the Lewis Structure for CHCl3 - YouTube. Touch device users explore by touch or with swipe gestures. Phosphorus trichloride is a inorganic compound with the chemical formula PCl 3.
How to Draw the Lewis Structure for CHCl3 - YouTube. TeCl4 Lewis Structure. For the central atom what is the formal charge.
Choose a central atom and draw a skeletal structure- Sketch a skeletal of the molecule with only single bonds. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds. It is toxic and reacts violently with water to release hydrogen chloride.
Lewis Structure of PCl5 Lewis structure of a compound is the arrangement of its underlying atoms valence shell electrons. Please watch the following video on how to draw Lewis structures. A colorless liquid when pure it is an important industrial chemical being used for the manufacture of phosphites and other organophosphorus compounds.
Chemistry learning made easyThis tutorial will help you deal with the lewis structure and moleculargeometry for boron triiodide BI3. Where V 7 5 7 6 7 32 V is the number of valence electrons of the POCl3molecule. So little structure H.
Here Phosphorous 5 valence electrons Chlorine 7 valence electrons 3 Cl 73 21 So total valence electrons 26. Which of the compounds have a linear molecular structure. Hydrogen has one valence electron but there are four of them.
What is the geometric shape. You know what for D s F two to impairs on sulfur. For the PCl5 Lewis structure we first count the valence electrons for the PCl5 molecule using the periodic table.
When drawing the Lewis Structure for this molecule and for drawings on future quizzes and exams it is ok if we draw the Cl atoms equally spaced around the Te atom and add the two lone electrons next to the Te atom or do we have to represent the electron repulsion from the lone pairs by drawing the Cl atoms a little. To draw the lewis structure first of all we need to sum up the valence electrons of all the atoms. Lewis structures make the use of dots to represent electrons and bonds between different electrons are represented through a straight line marked at the end of which is a set of electrons.
When autocomplete results are available use up and down arrows to review and enter to select. Which in turn enjoy many applications in herbicides insecticides plasticisers oil additives and flame retardants. It is a volatile liquid that reacts with water and releases HCl gas.
Hydrogen really needs to. Carbon has four each. What is the hybrid orbital designation for the central atom.
Se hace tiene que cumplir el octete osea que el P tenga 8 electrones para esto se hace el paso por paso cada linea -. Which of the compounds exhibit at least one bond angle that is approximately 120 degrees. The simple Lewis structure that is carbon in the center with eight valence electrons all used as bonding.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Drawing the Lewis Structure for PCl 5. Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms having a chemical formula of PCl3.
Draw the Lewis structures for SeO 2 PCl 3 NNO COS and PF 3. For the last one we have ch four. Find the total number of valence electrons in a molecule- Adding up the valence electrons of all the atoms in a molecule is the first step.
Which of the compounds exhibit sp 3 hybridization by the central atom. Step 3 4. How many sigma bonds in the formula.
Underneath draw the lewis structure. Answer all questions related to the drawing. Now lets see the proper steps to draw a lewis structure-1.
Paris bonding one hydrogen to carbon. LilNeg LilNeg P tiene 5 electrone. Which of the compounds are polar.
Estructura de Lewis de PCl3 1 Ver respuesta jisus2004 está esperando tu ayuda. Each chlorine has 63 lone pairs.

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Ch2cl2 Lewis Structure Dichloromethane Youtube
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

How To Draw The Lewis Structure Of Pcl5 Phosphorus Pentachloride Youtube

Lewis Dot Structure For Alf4 And Molecular Geometry Youtube

Is Pcl3 Non Polar Or Polar Why Quora

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube
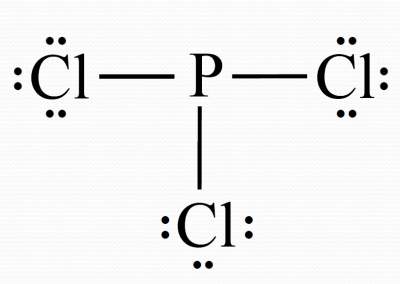
Pcl3 Phosphorus Trichloride Lewis Structure

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube
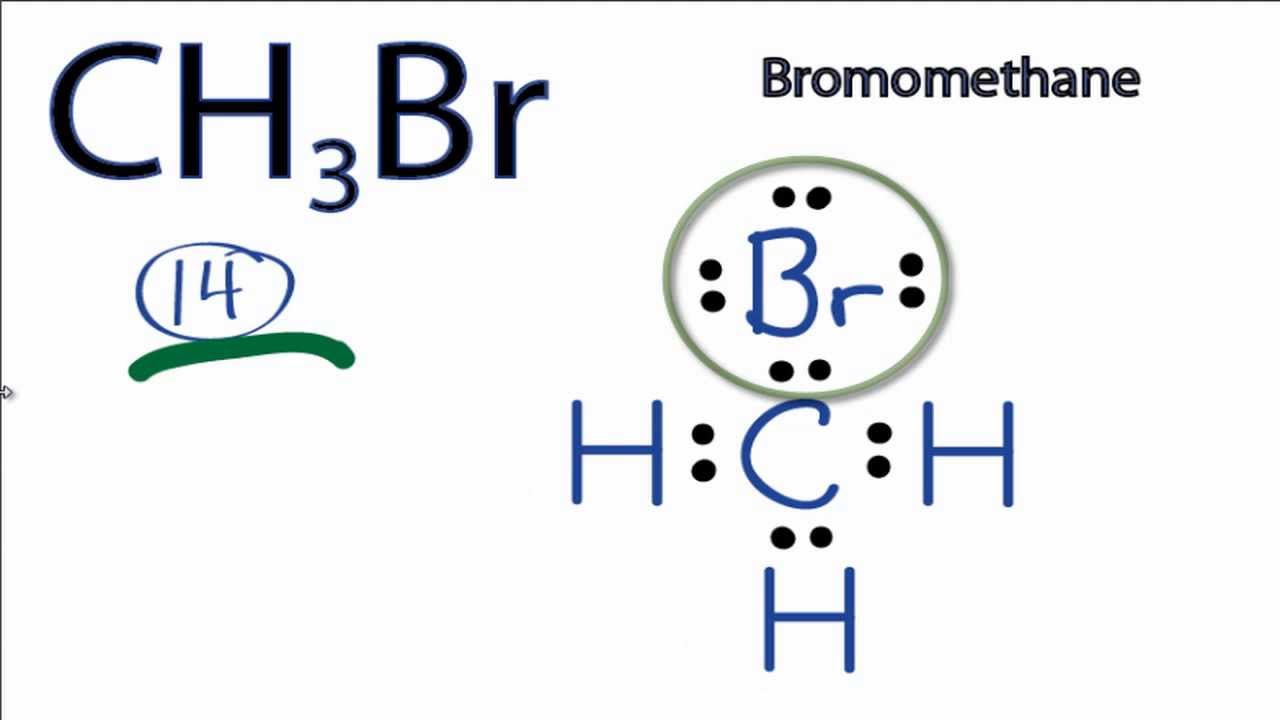
Ch3br Lewis Structure How To Draw The Lewis Structure For Ch3br Bromomethane Youtube

Is Pcl3 Polar Or Nonpolar Techiescientist

Pcl3 Phosphorus Trichloride Lewis Structure

How Is The Electron Dot Structure Of Pcl3 Determined Quora
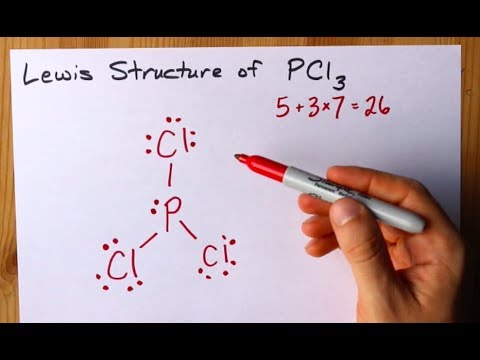
How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube

Pcl3 Molecular Geometry Shape And Bond Angles Youtube

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

Http Www Youtube Com Watch V Qojoaosk5ui Lewis Chemistry Math Equations
Is Pcl3 Non Polar Or Polar Why Quora