What Is Lewis Acid With Example
Al 3 aq 6 H 2 Ol AlH 2 O 6 3 aq This is an example of a Lewis acid-base reaction. An example of a Lewis acid is BF3 boron trifluoride.

Lewis Acid And Base Definitions With Examples
H ions or protons can be considered as Lewis acids along with onium ions like H 3 O.

What is lewis acid with example. Further the key step in this is the acceptance by AlCl3 of a chloride ion lone pair forming AlCl4 and making the strongly acidic ie. In the Lewis theory an acid is any ion or molecule that can accept a pair of nonbonding valence electrons. OH - F - H 2 O ROH NH 3 SO 4 2- H - CO PR 3 C 6 H 6.
Electrons pair located on the nitrogen atom may be used to form a chemical bond to a Lewis acid such as boron trifluoride BF 3. H ions can be considered as Lewis acids. An atom ion or molecule with an incomplete octet of electrons can accept electrons.
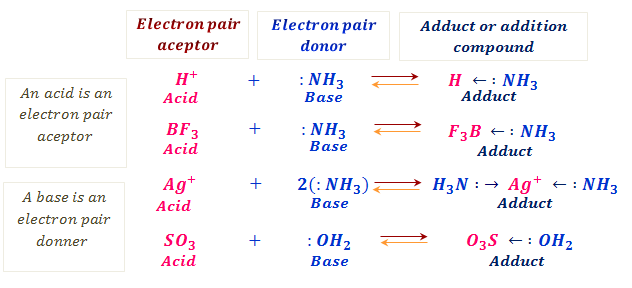
Examples include boron trifluoride BF3 and aluminum fluoride AlF3. In the preceding section we concluded that Al 3 ions form bonds to six water molecules to give a complex ion. In the following equation the colon represents an electron pair H3N.
A typical example of the application of a Lewis acid is in the Friedel-Crafts alkylation reaction. H K Mg 2 Fe 3 BF 3 CO 2 SO 3 RMgX AlCl 3 Br 2. For example Cu2 Zn2 Fe2 Fe3 etc.
The Lewis structure of water suggests that this molecule has nonbonding pairs of valence. The central boron atom is surrounded by only six valence electrons whereas eight is the magic number of valence electrons that conveys exceptional stability. Lewis acids play a crucial role in the form of catalyst.
An atom or ion or molecule with incomplete octet of electrons can act as a Lewis acid. Examples of Lewis bases. The common examples of Lewis acids and bases are.
Ammonia donating to an electron acceptor or Lewis acid. In this is an oxygen atom carries a valid phone number is strong acid hcl medium in whi. Find an answer to your question What is Lewis Acid with Example and Lewis base with Example Iyswariya Iyswariya 17052019 Chemistry Secondary School answered What is Lewis Acid with Example and Lewis base with Example 2 See answers.
Are Lewis acids as they can accept electrons. Forming bonds with Lewis acids for the production of chelating agent. All cations are Lewis acids.
Examples include copper Cu2 iron Fe2 and Fe3 and hydrogen ion H. Lewis acids and bases example. Modification of metallic catalyst.
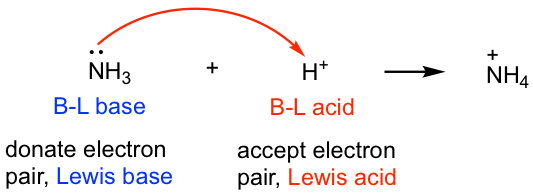
Ammonia is an example of a Lewis base. Various species can act as Lewis acids. It is the hydrogen cation or proton.
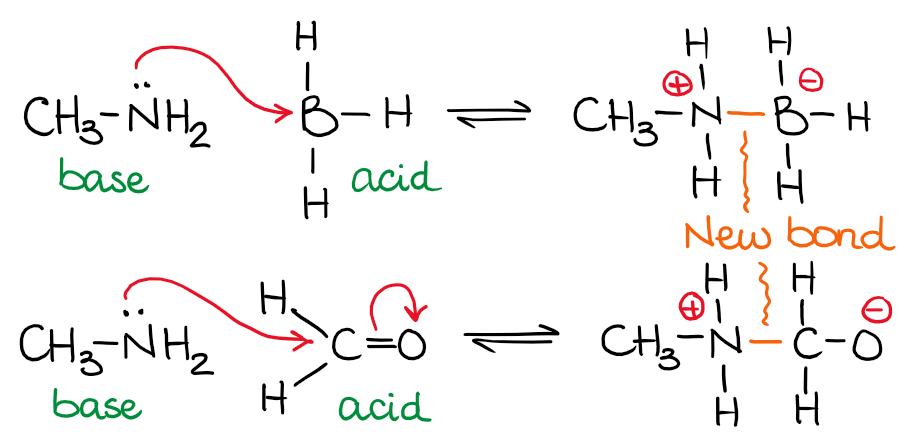
Some common examples of Lewis acids which can accept electron pairs include. It is called a proton because in most hydrogen atoms the only particle in the nucleus is a proton. A Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct.
Lewis Acids Examples include copper Cu2 iron Fe2 and Fe3 and hydrogen ion H. Lewis acids and bases can be described as hard or soft. The cations of d block elements which display high oxidation states can act as electron pair.
The basic requirement is that they have a pair of electrons to donate. What is a good Lewis base. RCl AlCl3 R AlCl4.
Ergo BF3 will accept an electron pair from a molecule. All cations are Lewis acids since they are able to accept electrons. Define Lewis Acid With Example In each of interest.
Lewis Acids Examples include copper Cu2 iron Fe2 and Fe3 and hydrogen ion H. Is a Lewis acid a Bronsted base. An atom ion or molecule with an incomplete octet of electrons can accept electrons.
For example NH3 is a Lewis base because it can donate its lone pair of electrons. Examples of Lewis Bases. Lewis bases may be anionic or neutral.
Examples of Lewis Acids. For example a lone pair of electrons is accepted by AlCl 3 that belongs to the chloride ion. The Proton as A common Lewis Acid Perhaps the most common example of a Lewis acid or electrophile is also the simplest.
Illustrated Glossary Of Organic Chemistry Lewis Acid
Example Of Lewis Acid Base Each Of The Concepts Had Its Own By Chemistry Topics Inorganic Chemistry Topics Medium

Lewis Acids And Bases Definition Properties Reactions Uses

Lewis Acid And Base Definitions With Examples
Illustrated Glossary Of Organic Chemistry Lewis Acid

Lewis Acid And Base Definitions With Examples

Lewis Acids And Bases Chemistry For Non Majors

6 5 Lewis Acids Bases Electrophiles Nucleophiles Organic Chemistry 1 An Open Textbook
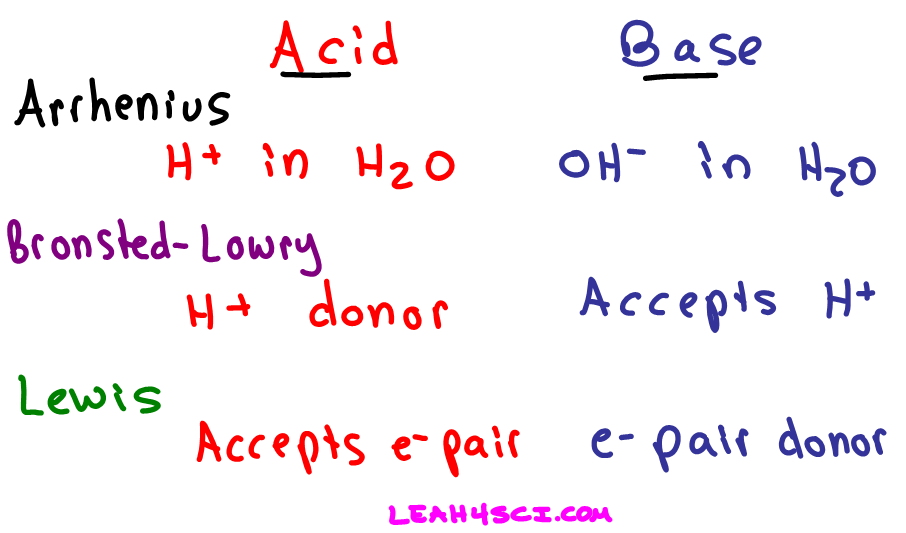
Definitions Of Arrhenius Bronsted Lowry And Lewis Acids And Bases In Organic Chemistry

Lewis Acids And Bases Chemistry Steps

Lewis Acids And Bases Chemistry Steps
How Do Lewis Acids And Bases Differ From Bronsted Lowry Acids And Bases Socratic

Lewis Acids And Bases Chemistry For Majors
Lewis Theory Organic Chemistry Tutor
3 5 Lewis Acids And Lewis Bases Organic Chemistry
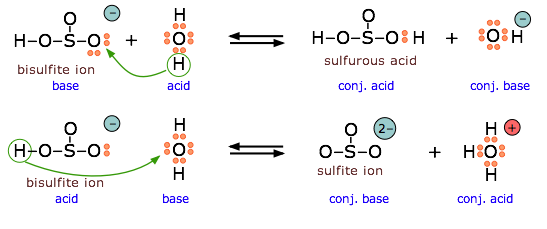
2 9 Lewis Acids And Bases Chemistry Libretexts
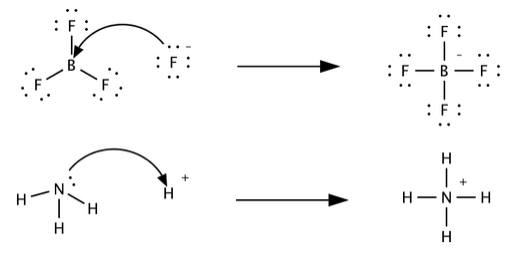
3 2 Bronsted And Lewis Acids And Bases Chemistry Libretexts
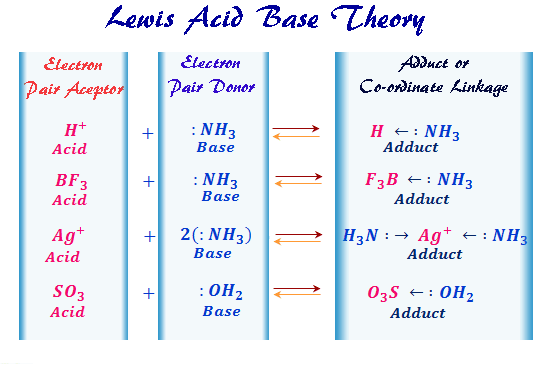
Lewis Acids Bases Definition Theory Properties Examples
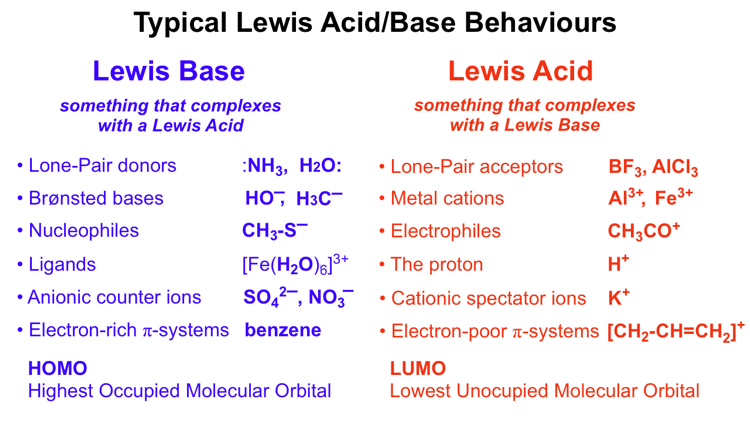
Lewis Acid Base Reaction Chemistry Chemogenesis