What Is The Lewis Structure Of H3o+
Oxonium is an oxygen. Bond Pair e- 8.

H3o Lewis Structure Hydronium Ion Youtube
8cdot22cdot1 18 Total sharedbonding electrons.

What is the lewis structure of h3o+. The molecular shape of H3O is a trigonal pyramid and electronic geometry is tetrahedral. I quickly take you through how to draw the Lewis Structure of hydronium ion H3O. As a result H3O ion is formed which is known as hydronium ion.
After that count the electrons and assign remaining lone pair electrons and the positive charge will be assigned over central atom. The H ion attacks on lone pair of oxygen atom in H2O and forms coordinate covalent with oxygen where oxygen is the donor. 7 rows Molecular Weight.
From the above chart we can see that hydronium ion is a AX3E type molecule A central atom X bonded atom E lone pair on A. -0 ---öc-º-H -H-2-3- H H -º-c-6-H I-O-I c H7. The HOH bond angle is approximately 113 and the center of mass is very close to the oxygen atom.
The Lewis structure of the triatomic H2O molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms. Note that the sign in the. 6 rows There are 8 valence electrons for the H3O Lewis structure.
I also go over hybridization shape and bond angle. The properly way to determine the Lewis structure based on this example is. The Lewis structure for PH3 is similar the the structure for NH3 since both P and N are in the same group on the Periodic table.
Lone Pairs around central atom 1. It is the reason why the bond angle that should have. From the above chart we will see that hydronium ion is a AX3E kind molecule A central atom X bonded atom E lone pair on A.
Because the base of the pyramid is made up of three identical hydrogen atoms the H 3O. So in keeping with the VSEPR chart H3O has trigonal pyramid as its molecular form and tetrahedral as its electron geometry. Moreover these bonds leave two lone pairs of electrons on the oxygen atom that mainly contributes to the tetrahedral bent geometrical structure of the H2O molecule.
The PH3 Lewis structure has 8 valence electrons. Draw and explain the Lewis structure for eqH_3O eq. Lone Pair e- 8 8 0.
The molecular shape of H3O is a trigonal pyramid and electronic geometry is tetrahedral. H3O is tetrahedral because when drawing the lewis structure there are a total of 8 electrons and so oxygen should have 3 bonds to hydrogens and then one lone pair which means there are four regions of electron density about the central atom which means the molecular geometry is tetrahedral but the shape is trigonal pyramidal. а 6 Two of the compounds below are the same while the third is a constitutional isomer.
It is the positive ion present when an Arrhenius acid is dissolved in water as Arrhenius acid molecules in solution give up a proton to the surrounding water molecules. Lewis structure for HOCl. It has a pyramidal shape like ammonia molecule.
The simplest covalent compound has two different atoms forming one covalent bond through a sharing of one valence. So according to the VSEPR chart H3O has trigonal pyramid as its molecular shape and tetrahedral as its electron geometry. Remember that hydrogen H only needs two valence electrons to have a full outershell.
Hydronium In chemistry hydronium is the common name for the aqueous cation H 3O the type of oxonium ion produced by protonation of water. Has a trigonal pyramidal molecular geometry with the oxygen atom at its apex. Lewis Structure of NH 4 Q 5 4 x 1 1 8.
What is the correct Lewis structure for acetic acid CH3CO2H. Lone Pairs Single or multiple bonds around the central atom 4. Which one is the constitutional isomer of the other two.
7 6 1 14 Total electrons needed for octetsdoublets. Correct answer to the question Draw the Lewis structure for the polyatomic hydronium H3O cation. The Lewis structure of H 3 O is.

Draw The Lewis Structure For H3o And State Its Molecular Geometry Is It Polar Or Nonpolar Study Com

Draw A Lewis Structure For H3o Show All Unshared Electrons And The Formal Charges If Any Assume Brainly Com

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube
Electron Dot Structure Of Hydronium Ion
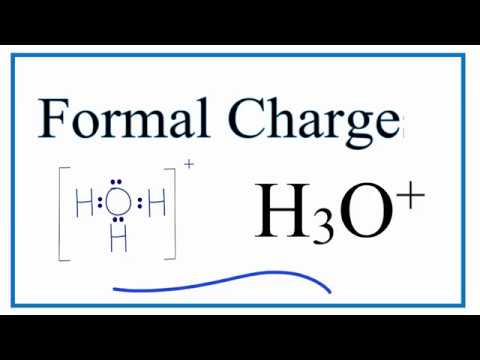
How To Calculate The Formal Charges For H3o Hydronium Ion Youtube

What Is The Hybridization Of O In The H3o Ion Study Com

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
What Is The Structure Of H3o Quora

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube

Electron Dot Structure Of Hydronium Ion Brainly In

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry
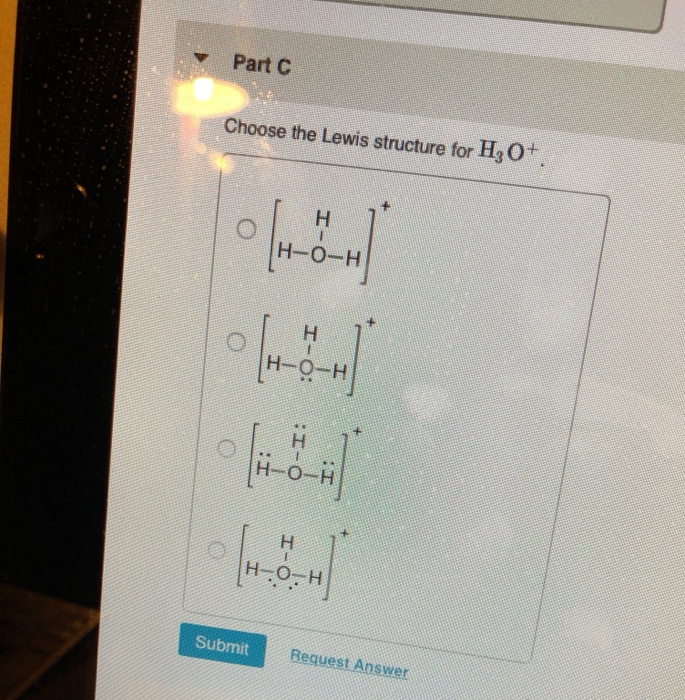
Part C Choose The Lewis Structure For H3o H O H Chegg Com

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

Chemistry Chemical Bonding 22 Of 35 Lewis Structures For Ions Hydronium Ion H3o Youtube

H3o Molecular Geometry Shape And Bond Angles Youtube

Draw A Lewis Structure For H3o Include All Hydrogen Atoms And Show All Unshared Electrons And The Brainly Com

