Xef2o Lewis Structure With Lone Pairs
Draw Lewis Structure for BrO3- The textbook answer involves a central Br atom with one lone electron pair and five shared pairs two double bonds and one single bonds. Asked by DEAR GOD SOMEONE HELP ME PLEASE on April 22 2017 chemistry Draw a lewis structure for BrO4- in which all atoms have the lowest formal changes.

Xef2o Lewis Structure Xenon Oxytetrafluoride Youtube
Experts are tested by Chegg as specialists in their subject area.
Xef2o lewis structure with lone pairs. XeF2 is a linear molecule due to the arrangement of fluorine atoms and the lone pairs of electrons in the symmetric arrangement. Put lone pairs on atoms. Afterwards the xenon atom will make double bond with oxygen atom to fulfill its octet.
A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure Xeon TetrafluorideFor the XeF4 structure use the periodic table to find the total n. Following questions about atomic fluorine oxygen and xenon as well as some of their provided draw the complete Lewis electron-dot diagram for each of the. The single lone pair gives the compound a seesaw shape which then would be polar.
Show all lone pairs. Draw a complete Lewis Structure showing all bonds and lone pairs for a molecule with the general formula AX4 if A was from group 4A and X was from group 7A. Again more two valence electrons of Xe atom will get bonded to the O atom.
Here is a picture of its structure. Elements in the first 2 periods of the Periodic Table do not have access to the d sublevel and must adhere to the octet or duet H and He rule. Please help me i dont know how to do this.
Determine the central atom in this molecule. There would be 3 Lone Pair LP electrons around F 2. Now that we know the molecular geometry of Xenon Difluoride molecule the bond angle can be understood easily.
ABOUT XeF2- It is a very good fluorination agent. To do so we first need to do the following steps. Draw a complete Lewis Structure showing all bonds and lone pairs for a molecule with the general formula AX4 if A was from group 4A and X was from group 7A.
Draw the lewis structure for. In doing so you can see that Xe has 5 electron densities with 4 bonded pairs and one lone pair. Draw the Lewis structure.
XeO2F2 is polar and it helps to identify this through drawing out the lewis structure. This gives Br twelve electrons rather than the typical eight. The xenon atom will bond with the four fluorine atoms via four single bonds which will ensure that each of the four fluorine atoms gets a complete octet.
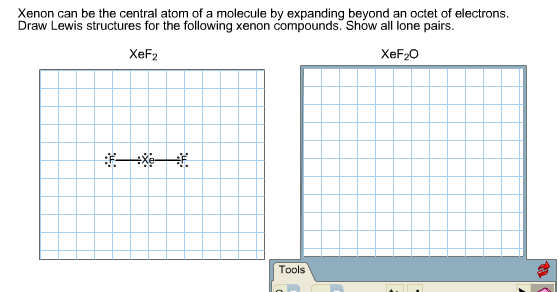
Draw the Lewis structure of xenon difluoride and give the number of lone pairs of electrons around the central atom 1. XeF 2 is dsp 3 hybridized and contains 3 lone pair and 2 bonding pairs of valence electrons around the Xenon. The structure of eqXeF_2 eq is shown.
We review their content and use your feedback to keep the quality high. But check the formal charges -- its probably not the be. This one is a bit tough since the first Lewis structure you generate will seem like the right one.
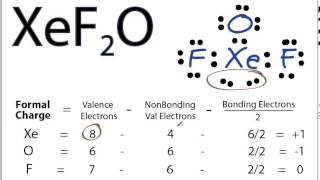
Include all the lone pairs Draw the structure of bromous acid HBrO2HBrO2. Check the stability and minimize charges on atoms by converting lone pairs to bonds until most stable structure is obtained. Xe would be your central atom O is attached to it by a double bond and your two Fs are attached be a single bond.
The remaining two electrons will remain as a lone pair. Show all lone pairs. Once we know how many valence electrons there are in XeF2 we can distribute them around the central atom and attempt to fill the outer shells of each atom.
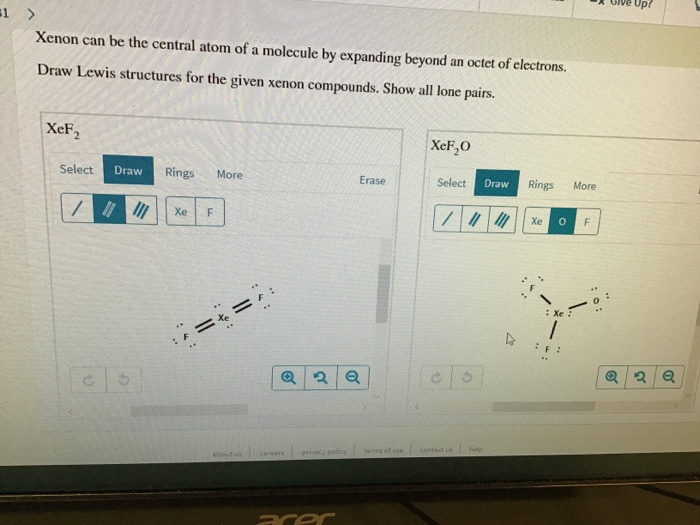
The central Xe atom is having 2 lone pairs in the molecule XeOF_2. Here in this molecule the Xenon Xe atom is the central atom which has total 8 valent electrons. Xenon can be the central atom of a molecule by expanding beyond an octet of electrons Draw Lewis structures for the given xenon compounds.
Were being asked to draw a Lewis structure for XeF 2 O. The lewis structures of both the given molecule will give the idea about the arrangement of electrons around these atoms as bonding pairs and lone pairs. XeF2 XeF2O Draw the Lewis structure of BeCl2BeCl2.
For the XeF2 Lewis structure we first count the valence electrons for the XeF2 molecule using the periodic table. We also need to show all lone pairs. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell.
Who are the experts. Lewis dot symbols provide a simple rationalization of why elements form compounds When drawing the Lewis structure. The VSEPR predicts the linear shape.
There are two pairs of bonded electrons and three lone pairs of electrons. So 2 of the valence electrons of Xe atom will get bonded to the two F atoms. Drawing the correct lewis structure is important to draw resonance structures correctly.
What is the lewis structure of XeF2O. Calculate the total number of valence electrons present.
Xef2o Lewis Structure How To Draw The Lewis Structure For Xef2o Youtube
Xenon Can Be The Central Atom Of A Molecule By Chegg Com
How To Draw An Xef2o Lewis Structure Quora
How To Draw Lewis Structure For Xef2 Drawing Easy

Answer Draw Lewis Structures For The Foll Clutch Prep
Calculating Co2 Formal Charges Calculating Formal Charges For Co2 Youtube

Xef2o Lewis Structure How To Draw The Lewis Structure For Xef2o Youtube

Draw Lewis Structure For Xef2o

On Feedback Xef Should Have A Total Of 8 2 7 Chegg Com

Live Up 1 Xenon Can Be The Central Atom Of A Chegg Com
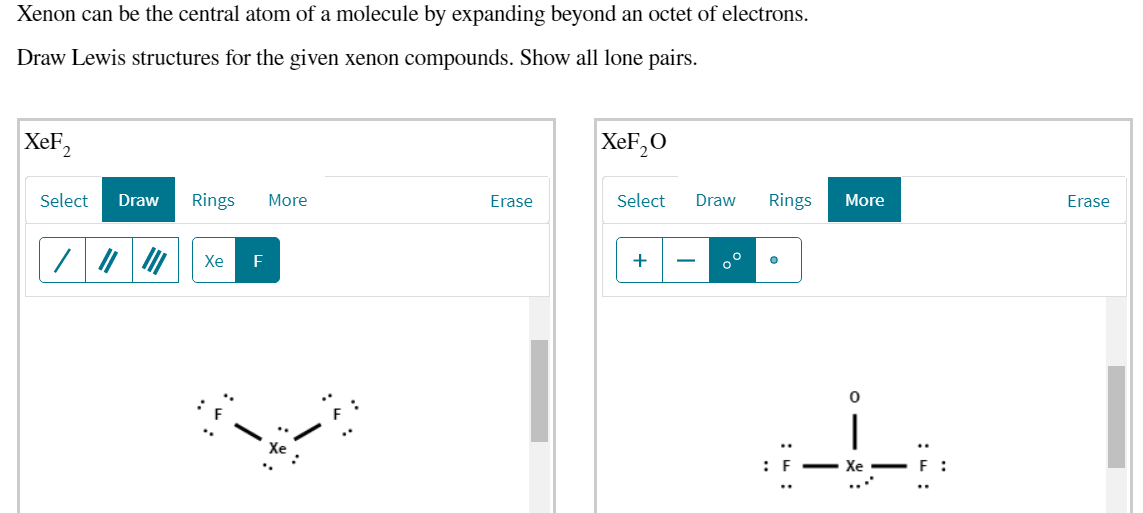
Xenon Can Be The Central Atom Of A Molecule By Chegg Com

Draw Lewis Structure For Xef2o

Solution What Is The Lewis Dot Structure Chemistry
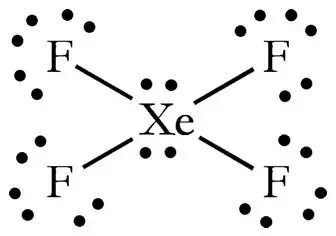
How Can The Lewis Structure For Xef4 Be Determined Quora

Xeo2f2 Lewis Structure How To Draw The Lewis Structure For Xeo2f2 Youtube

What Is The Lewis Structure For Cho2 Quora
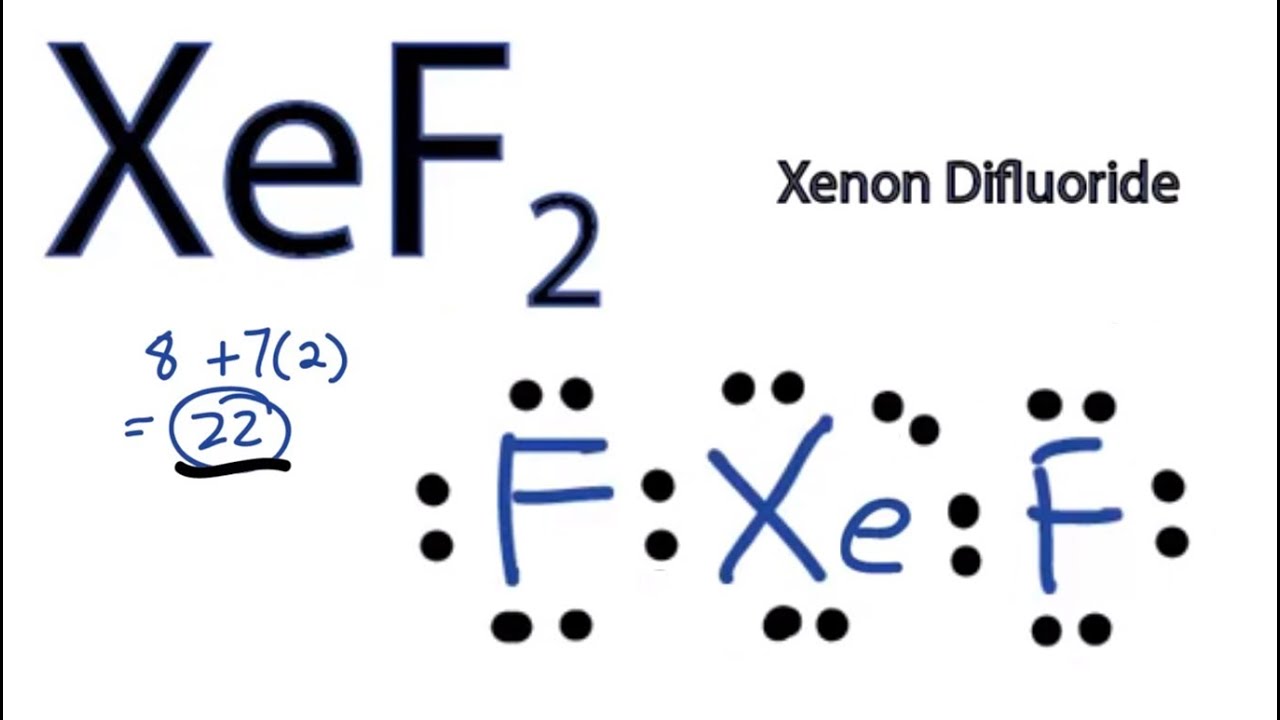
Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 Youtube

Xef2o Lewis Structure How To Draw The Lewis Structure For Xef2o Youtube

Xef2o Lewis Structure Xenon Oxytetrafluoride Youtube
