Pcl3 Lewis Structure Bond Angle
As per the molecular geometry of the molecule the bond angle of PCl3 should be 109 degrees. Total outermost valence shell electrons available for PCl3 Lewis structure dot structure 5 73 26 valence electrons in PCl3 calculation of total valence electron of PCl3 molecule Step-2.
Answer In Organic Chemistry For Tj 92420
Molecule PCl 3 CO 2 H 2 S Lewis structure b The following table shows the Lewis structures and bond angles for the molecules SO2 and H 2 CO.
Pcl3 lewis structure bond angle. One bond lies above the equatorial level and the other one lies below it. We can see there are 4 things around the central atom. The bond angle of NCl3 is 1071 as it slightly decreases because of the lone pair present on nitrogen that creates repulsion between bond pairs and lone pair hence causes to decrease in angle.
What is the Lewis dot structure for CH2O. PCl3 has a bond angle of 103 degrees. Chapter 14 Problem 79AE is solved.
A Draw the Lewis structure electron dot diagram for each of the following molecules. As a result the bond angle of Cl-P-Cl gets deviated and is less than 109 degrees. But as there is one lone pair of electrons on the central phosphorus atom the bond angle will reduce from 109 degrees because of the repulsive forces of the lone pair.
The remaining 3 bonds are equatorial in nature. PCl3 Molecular Orbital MO Diagram In a molecular orbital diagram of PCl3 we can see 3 bonding orbitals which will be occupied. Also there are no charges on atoms in PCl3 lewis structure.
Molecule SO 2 H 2 CO Lewis structure Approximate bond angle around the central atom 120o o120. Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond. Because of the electron pair the bond angle in PCl3 is.
Bond Angle Of PCL3 A bond angle is an angle between two bonds that include an atom and the bond angle is usually measured in degrees. The geometry of the BF 3 molecule is called trigonal planar see Figure 5. I quickly take you through how to draw the Lewis Structure of PCl3 phosphorous trichloride.
The fluorine atoms are positioned at the vertices of an equilateral triangle. Can be predicted if the Lewis structure of the molecule or the compound is known. The geometry of the CH3I molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory VSEPR Theory which states that molecules will choose the CH3I geometrical shape in which the.
Therefore the geometry of the electron pairs is tetrahedral with three of the angles occupied by the bonding electron pairs. Phosphorus forms three single bonds with three chlorine atoms and a double bond with an Oxygen atom. Both the bonds make an angle of 90 degrees with the plane.
If I talk about the bond distance so its the distance between the central part and the other two bonded atoms which is joining the center along a straight line. The 3 bonds lie in the same plane making an angle of 120 degrees with one another. DiOrio Julia The bond angles in the ammonia molecule are less than 109.
6 The bond angles are. The central atom of Sn is surrounded by a pair of unbound electrons and three single bonds. What shape is bf3.
Axial-axial 180. Because of the electron pair the bond angle in PCl3 is. Sncl3 Lewis Structure What is the molecular geometry of sncl3.
Phosphorus has sp3 hybridization in this molecule. We can see there are 4 things around the central atom. Lets draw the Lewis structure of PCl3.
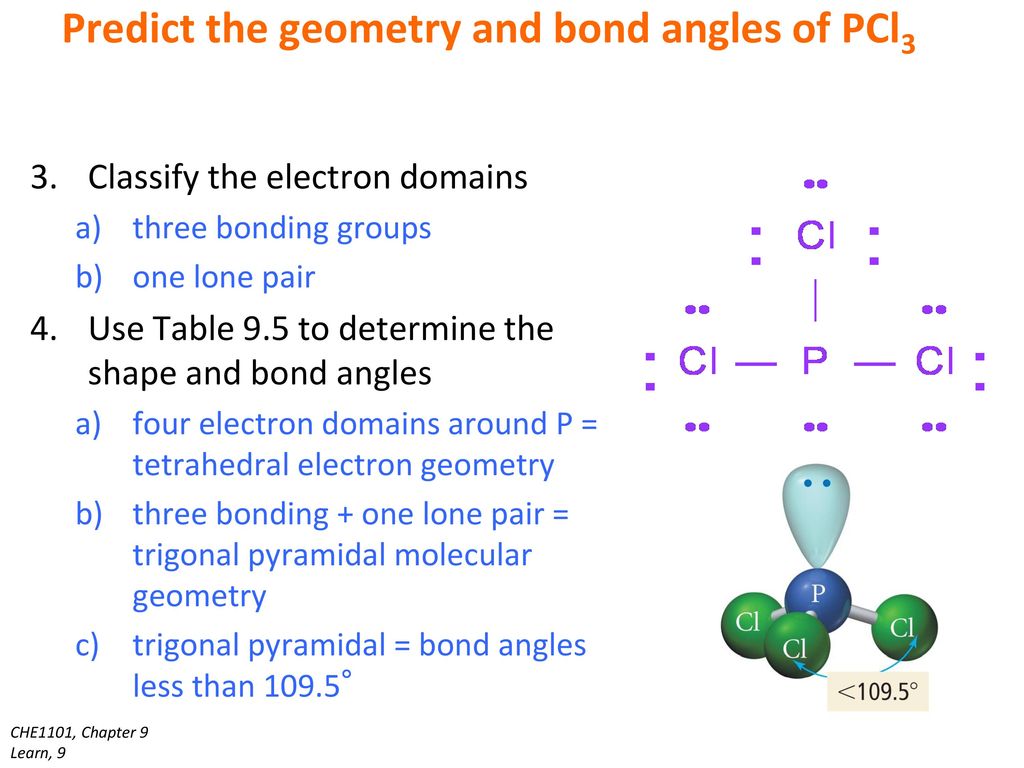
The decrease from the ideal bond angle of trigonal pyramidal compounds 109 degrees is due to repulsion between the lone pairs on phosphorus. 3 Chlorines and a lone pair of electrons. The bond angle of PCL3 is 109o.
SOLUTION a The Lewis structure of the SnCl3 ion looks like this. 3 Chlorines and a lone pair of electrons. The F-B-F angle is 120 and all four atoms lie in the same plane.
Locate the atom with the least electronegative charge and place it in the center of the PCl3 molecular geometry. The bond angles are approximately 1098 and the molecular geometry of this molecule is tetrahedral. Lewis Structure VSEPR Hybridization Last modified by.
Lets draw the Lewis structure of PCl3. I also go over hybridization shape and bond angle. The CH3I Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the CH3I molecule.
The various physical properties of a compound such as polarity shape hybridization of central atom bond angle etc. Is NCl3 polar or nonpolar.

How Many Lone Pair Electrons Are There In Pcl3 Quora

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have
Is Pcl3 Non Polar Or Polar Why Quora
Your Turn A Central Atom Has Two Lone Pair Of Electrons Around It And Two Single Bonds To Other Atoms What Is The Electron Pair Geometry Around The Central Ppt Download

What Is The Bond Angle Of Pcl3 Quora

Pcl3 Lewis Structure And Molecular Geometry Youtube

How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
Answer In Organic Chemistry For Tj 92420
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

What Is The Molecular Shape Of Pcl3 Quora

Pcl3 Molecular Geometry Shape And Bond Angles Youtube

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist