Phosphorus Pentachloride Lewis Structure
CS 2 - Carbon Disulfide 11. 10 times the heat of evaporation keeps the system at its boiling point and the phosphorus trichloride distills off.

Chemistry291 Hand Note Ehene To Polyethene Conversion Or Reaction What Is Chemistry Chemical Equation Organic Chemistry
PCl 5 phosphorus pentachloride.

Phosphorus pentachloride lewis structure. H only needs 2 valence electrons. NH 4 Lewis Structure. Valence diagrams of a compound represent the connectivity of the elements lines between two elements sometimes called bonds represented a saturated valency for each element.
Phosphorus pentachloride is manufactured by either batch or continuous processing. AsF 5 - Arsenic Pentafluoride 14. NOCl - Nitrosyl Chloride 15.
Chlorine as it has a valence of one can be substituted for hydrogen so phosphorus has a valence of 5 in phosphorus pentachloride PCl 5. S and P sometimes have more than 8 val. POOH 3 - Phosphoric Acid.
Be and B dont need 8 valence electrons. SeF 6 - Selenium Hexafluoride 13. 3 PCl 5 5 AsF 3 3 PF 5 5 AsCl 3 Structure.
Notable Exceptions to the Octet Rule. What are all of the possible ClPCl bond angles. Phosphorus pentachloride is the chemical compound with the formula PCl 5It is one of the most important phosphorus chlorides others being PCl 3 and POCl 3PCl 5 finds use as a chlorinating reagent.
ICl 5 - Iodine Pentachloride 6. SO 4 2- N 2 O XeO 3. Phosphorus pentafluoride was first prepared in 1876 by the fluorination of phosphorus pentachloride using arsenic trifluoride which remains a favored method.
The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorusThe heat of reaction ca. It is a colourless water-sensitive and moisture-sensitive solid although commercial samples can be yellowish and contaminated with hydrogen chloride. 90 120 and 180 e.
In the former the phosphorus trichloride usually dissolves in carbon tetrachloride before being treated with chlorineA mixture of about one part of phosphorus trichloride to one part of carbon tetrachloride is introduced to a water-jacketed vessel that contains an efficient stirrer and a tight cover with a. Single-crystal X-ray studies indicate that the PF 5 has trigonal bipyramidal geometry. H 3 O Lewis Structure.
A molecules shape strongly affects its physical properties and the way it interacts with other molecules and plays an important role in the way that biological molecules proteins enzymes DNA etc. Check the Formal Charges to make sure you have the best Lewis Structure. XeCl 2 - Xenon Dichloride 8.
Find 7719-12-2 and related products for scientific research at MilliporeSigma. Drawing a Lewis structure is the first steps towards predicting the three-dimensional shape of a molecule. The phosphorus pentachloride molecule is nonpolar and contains no lone unshared electron pairs on the phosphorus atom.
Thus it has two distinct types of PF bonds. BeI 2 - Beryllium Diiodide 12. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
In a Hoechst continuous process molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride. PCl 5 - Phosphorus Pentachloride 7. AlCl 3 - Aluminum Trichloride 10.
XeF 4 - Xenon Tetrafluoride 9.

Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry

P Cl Pcl Balanced Eq Phosphorus Chlorine Gas To Form Phosphorus Pentachloride Balanced Equation Youtube Balance Phosphorus Chlorine

Pin On Chemistry What Is Chemistry

Phosphorus Pentachloride Lewis Dot Structure Google Search Molecular Geometry Covalent Bonding Molecular
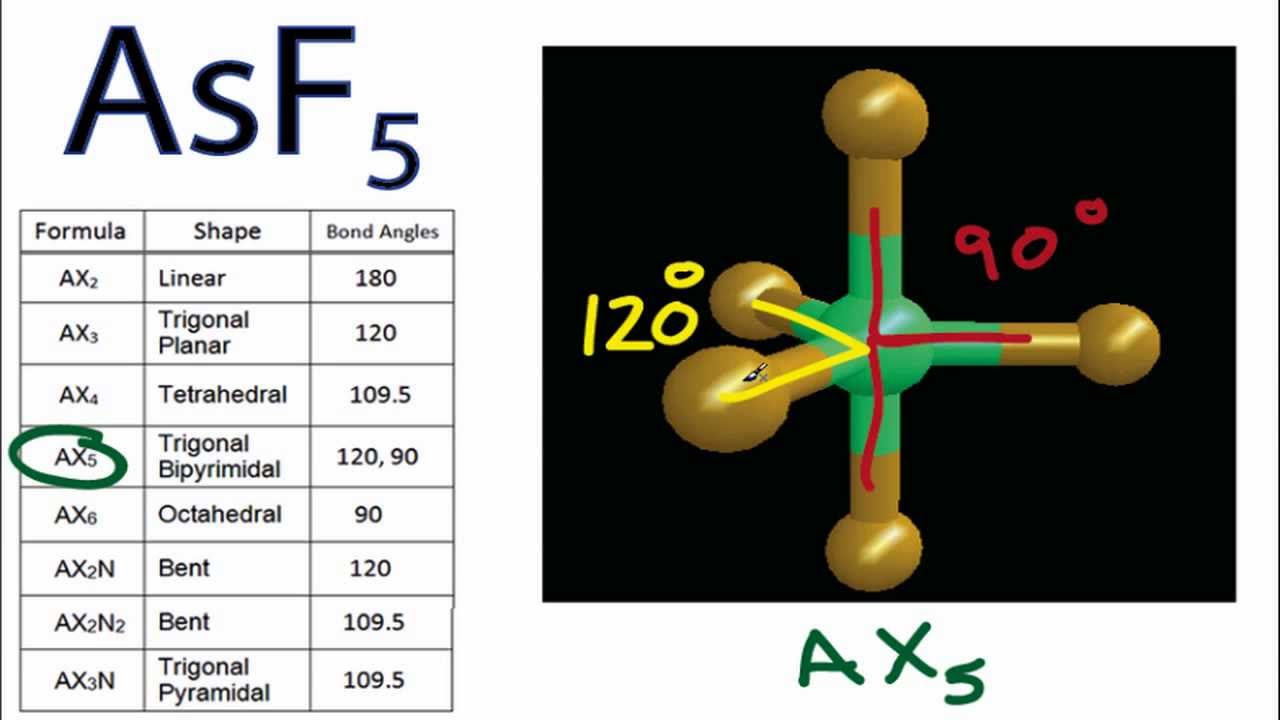
Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry
