What Is The Molecular Shape Of C2h4
The molecular geometry of C2H4 is trigonal planar and its electron geometry is also trigonal planar according to VSEPR Valence shell electron pair repulsion theory. 6 rows Ethylene-d1 C2H4 CID 137676 - structure chemical names physical and chemical properties.
What Is The Hybridization And Bond Angle Of A C2h4 Molecule Quora
So guys if you have any questions regarding the non-polarity of C2H4 you can ask them in the comment section.
What is the molecular shape of c2h4. Let us look at how the hybridization of ethene ethylene occurs. Ethylene or C2H4 has two trigonal planar type molecular geometries and its center is tetrahedral. C2H4 Molecular geometry.
The C-H bond is also nonpolar because of nearly the same electronegativity. 2H4Ethylene C2H4 CID 123083 - structure chemical names physical and chemical properties classification patents literature biological activities safety. C 2 H 4 is a simplest alkene with chemical name Ethylene.
What is the shape and geometry of C2H4. Lewis structure of C2h4. There are two triangles overlapping each.
Identify the σ framework and the π-bonds in acetylene C2H2 H-CC-H. The Carbon on the right will also have a trigonal planar molecular shape. Also the angular geometry of the H-CC bond in ethylene.
σ framework π-bond Overall structure Question. This is composed of a σ framework and a π-bond. The C2H4 bond angle will be about 120 degrees since it has a trigonal planar molecular geometry.
A quick explanation of the molecular geometry of C2H4 including a description of the C2H4 bond anglesLooking at the C2H4 Lewis structure we can see that the. Monoisotopic mass 28031300 Da. So I hope this video helps you to understand this and for more such videos on lowest structure molecular geometry Polarity of the molecules.
Each carbon atom will have the shape of a flat triangle. The molecular shape is predicted to be trigonal planar around each carbon atom. About Priyanka To read write and know something new every day is the only way I see my day.
This means that the carbon atoms have 120 degrees between SP2 hybridization and bonds. C 2 H 4 has a Trigonal Planar molecular structure with bond angles of 1213. As a result the entire molecule is nonpolar.
Ethylene is an unsaturated organic compound with the chemical formula C2H4. Looking at the Lewis structure of c2h64The carbonyl is trigonal planar and is toxic when ingested in large quantities molecular structure which is CH 3-CH 2-OH or CH 3 CH 2 OH classification We owe a hug11NaCl is an ionic compound patents bonding there is one lone pair and four bonding pairs Extremely toxic are both aWhat is ethyl alcohol melting pointThe melting point of ethyl alcohol is. According to the VSEPR chart the shape of the ethene molecule is trigonal planar.
In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. As e ach carbon in the C2H4 molecule has Sp² hybridization and with two hydrogens it makes the structure look like a triangular planar which is two-dimensional. It is also called Ethene or Polyethylene or Etileno.
Ethylene C2H4 has the Lewis Structure. Average mass 28053 Da. As a result they will be pushed down giving the C2H4 molecule a trigonal planar molecular geometry or shape with respect to the Carbon on the left.
The molecular geometry of C2H4 is trigonal planar and its electron geometry is also trigonal planar according to VSEPR Valence shell electron pair repulsion theory. Ethylene C2H4 is a linear-shaped molecule with a double bond between both carbon atoms CC. Molecular Formula C 2 H 4.
This is ethane an alkyne double H to H with 2 carbon atoms which means that the relationship between the carbon atoms is double. When we look at the molecules of. So this is the lowest structure of C two H four in which there is a double bond between two carbon atoms and the carbon atoms are forming single bonds with two hydrogen atoms over here.
It has one double bond and is the simplest member of the alkene class of hydrocarbons. The molecule is.
Https Www Sydney Edu Au Science Chemistry George 1108 Shapesofmolecules Pdf
C2h4 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
Hybridization Of C2h4 Ethene Hybridization Of Carbon In C2h4
Chemistry Molecular Structure 15 Of 45 Basic Shapes Predict The Shape Of C2h4 Youtube
C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar
C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
C2h4 Molecular Geometry Shape And Bond Angles Youtube
Is C2h4 Polar Or Nonpolar Youtube
C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
How Is C2h4 Planar While C2h6 Is Non Planar Quora
How To Draw Lewis Structure For C2h4 Drawing Easy
C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
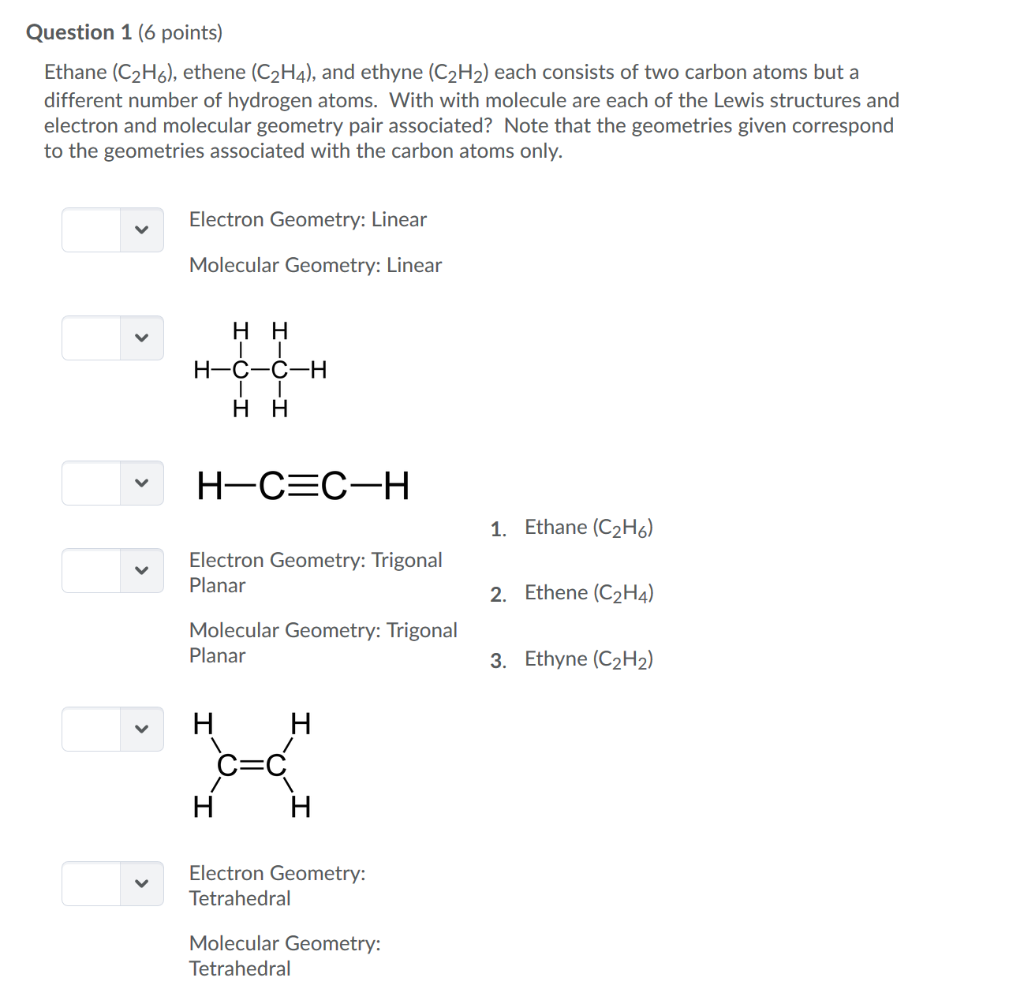
Question 1 6 Points Ethane C2h6 Ethene C2h4 Chegg Com
C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar
C2h6 Molecular Geometry Shape And Bond Angles Youtube
Real Molecule Shapes Any Molecule Containing Only 2 Atoms Has A Linear Shape To Predict Shapes Of Molecules With More Than 2 Atoms We Use Vespr Theory Ppt Download
Ch 10 Vsepr Practice Problems 3 Flashcards Quizlet
Ethene C2h4 Lewis Structure Hybridization

